Lead stearate
Appearance
| |
| Names | |
|---|---|
| Other names
Lead(2+) octadecanoate, lead(II) stearate, lead distearate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.733 |
| EC Number |
|
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C 36H 70PbO 4 | |
| Molar mass | 774.14 |
| Appearance | White powder |
| Density | 1.4 g/cm3 |
| Melting point | 115.7 °C (240.3 °F; 388.8 K) |
| Boiling point | 359.4 °C (678.9 °F; 632.5 K) |
| Slightly soluble | |
| Hazards | |
| GHSlabelling: | |
  
| |
| Danger | |
| H302,H332,H360,H373 | |
| P260,P261,P281,P304,P340,P405,P501 | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
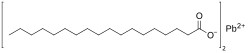
Lead stearateis ametal-organic compound,a salt ofleadandstearic acidwith the chemical formulaC
36H
70PbO
4.[1]The compound is classified as ametallic soap,i.e. a metal derivative of afatty acid.[2]The compound is toxic.
Synthesis
[edit]The compound can be prepared by reactingstearic acid,lead(II) oxide,and a catalystacetic acid.[3]
Also, an exchange reaction betweenlead(II) acetateandsodium stearate:
Physical properties
[edit]White powder with a slight fatty odor. Sinks in water.[4]Hygroscopic in air.
Slightly soluble in water.[1]Soluble in hotethanol.
Uses
[edit]The compound is used as adrierin oil paints and varnishes to speed the polymerization and oxidation processes. Also used as alubricantand stabilizer in vinyl polymers and as acorrosion inhibitorin petroleum products.[5][6][7]
References
[edit]- ^ab"Lead Stearate".American Elements.Retrieved7 March2023.
- ^"T3DB: Lead stearate".t3db.ca.Retrieved7 March2023.
- ^"Preparation process of lead stearate based on melting method".18 December 2013.Retrieved7 March2023.
- ^"LEAD STEARATE | CAMEO Chemicals | NOAA".cameochemicals.noaa.gov.Retrieved7 March2023.
- ^"Lead Stearate » Waldies Co. Ltd".Waldies Co. Ltd.Retrieved7 March2023.
- ^Encyclopedia of Chemical Technology: Fuel resources to heat stabilizers.Wiley. 1991. p. 1074.ISBN978-0-471-52669-8.Retrieved7 March2023.
- ^Titow, M. V. (6 December 2012).PVC Technology.Springer Science & Business Media. p. 269.ISBN978-94-009-5614-8.Retrieved7 March2023.


