Lesch–Nyhan syndrome
This articleneeds additional citations forverification.(February 2022) |
| Lesch–Nyhan syndrome | |
|---|---|
| Other names | Juvenile gout,[1]Primary hyperuricemia syndrome, Choreoathetosis self-mutilation syndrome, X-linked primary hyperuricemia, HGPRT deficiency |
 | |
| A boy with Lesch–Nyhan syndrome wearing arm restraints | |
| Specialty | Endocrinology |
| Symptoms | self harm,dystonia,chorea,spasticity,intellectual disability,hyperuricemia, |
| Complications | kidney failure,megaloblastic anemia |
| Differential diagnosis | cerebral palsy,dystonia,famillial dysautonomia |
| Frequency | 1 in 380,000[2] |
Lesch–Nyhan syndrome(LNS) is a rareinherited disordercaused by a deficiency of theenzymehypoxanthine-guanine phosphoribosyltransferase(HGPRT). This deficiency occurs due tomutationsin theHPRT1genelocated on theX chromosome.LNS affects about 1 in 380,000 live births.[3]The disorder was first recognized and clinically characterized by American medical studentMichael Leschand his mentor, pediatricianWilliam Nyhan,atJohns Hopkins.[4]
The HGPRT deficiency causes a build-up ofuric acidin all body fluids. The combination of increased synthesis and decreased utilization ofpurinesleads to high levels of uric acid production. This results in bothhigh levels of uric acid in the bloodandurine,associated with severegoutand kidney problems. Neurological signs include poor muscle control and moderateintellectual disability.These complications usually appear in the first year of life. Beginning in the second year of life, a particularly striking feature of LNS isself-mutilatingbehaviors, characterized by lip and finger biting. Neurological symptoms include facial grimacing, involuntary writhing, and repetitive movements of the arms and legs similar to those seen inHuntington's disease.Thecauseof the neurological abnormalities remains unknown. Because a lack of HGPRT causes the body to poorly utilizevitamin B12,some males may developmegaloblastic anemia.[5]
LNS is inherited in anX-linked recessivemanner; the gene mutation is usually carried by the mother and passed on to her son, although one-third of all cases arisede novo(from new mutations) and do not have a family history. LNS ispresent at birthin baby boys. Most, but not all, persons with this deficiency have severe mental and physical problems throughout life. Cases in females are very rare.[6]
The symptoms caused by the buildup of uric acid (goutandkidneysymptoms) respond well to treatment with medications such asallopurinolthat reduce the levels of uric acid in the blood. The mental deficits and self-mutilating behavior do not respond well to treatment. There is no cure, but many affected people live to adulthood. Several new experimental treatments may alleviate symptoms.
Signs and symptoms
[edit]LNS is characterized by three major hallmarks:neurologicdysfunction,cognitiveandbehavioraldisturbancesincluding self-mutilation, anduric acidoverproduction (hyperuricemia). Damage to thebasal gangliacauses affected individuals to adopt a characteristic fencing stance due to the nature of the lesion. Some may also havemacrocytic anemia[7]due to the faulty DNA synthesis, most likely due to deficient purine synthesis that leads to a lag of cell division with respect to increases in cell mass.[8][9]Virtually all patients are male; males experience delayed growth andpuberty,and most develop shrunken testicles ortesticular atrophy.Female carriers are at an increased risk forgouty arthritisbut are usually otherwise unaffected.[2]
Overproduction of uric acid
[edit]One of the first symptoms of the disease is the presence of sand-like crystals ofuric acidin the diapers of the affected infant. Overproduction of uric acid may lead to the development of uric acid crystals or stones in thekidneys,ureters,orbladder.Such crystals deposited in joints later in the disease may producegout-likearthritis,with swelling and tenderness. The overproduction of uric acid is present at birth, but may not be recognized by routine clinical laboratory testing methods. The serum uric acid concentration is often normal, as the excess purines are promptly eliminated in the urine. The crystals usually appear as an orange grainy material, or they may coalesce to form either multiple tiny stones or distinct large stones that are difficult to pass. The stones, or calculi, usually causehematuria(blood in the urine) and increase the risk ofurinary tract infection.Some affected people have kidney damage due to suchkidney stones.Stones may be the presenting feature of the disease, but can go undetected for months or even years.[10]
Nervous system impairment
[edit]The periods before and surrounding birth are typically normal in individuals with LNS. The most common presenting features are abnormally decreasedmuscletone (hypotonia) and developmental delay, which are evident by three to six months of age. Affected individuals are late in sitting up, while most never crawl orwalk.[11]
Irritability is most often noticed along with the first signs of nervous system impairment. Within the first few years of life,extrapyramidalinvolvement causes abnormal involuntary muscle contractions such as loss of motor control (dystonia), writhing motions (choreoathetosis), and arching of the spine (opisthotonus). Signs ofpyramidal systeminvolvement, including spasticity, overactive reflexes (hyperreflexia) and extensorplantar reflexes,also occur.[12]The resemblance to athetoidcerebral palsyis apparent in the neurologic aspects of LNS. As a result, most individuals are initially diagnosed as having cerebral palsy. The motor disability is so extensive that most individuals never walk, and become lifelong wheelchair users.[13]
Self-injuring behavior
[edit]Persons affected are cognitively impaired and have behavioral disturbances that emerge between two and three years of age. The uncontrollableself-injuryassociated with LNS also usually begins at three years of age. The self-injury begins with biting of the lips and tongue; as the disease progresses, affected individuals frequently develop finger biting and headbanging.[14]The self-injury can increase during times of stress. Self-harm is a distinguishing characteristic of the disease and is apparent in 85% of affected males.[15]
The majority of individuals are cognitively impaired, which is sometimes difficult to distinguish from other symptoms because of the behavioral disturbances and motor deficits associated with the syndrome. In many ways, the behaviors may be seen as a psychological extension of the compulsion to cause self-injury, and include rejecting desired treats or travel, repaying kindness with coldness or rage, failing to answer test questions correctly despite study and a desire to succeed, and provoking anger from caregivers when affection is desired.[15]
Compulsive behaviors also occur, including aggressiveness,vomiting,spitting, andcoprolalia(involuntary swearing). The development of this type of behavior is sometimes seen within the first year, or in early childhood, but others may not develop it until later in life.[16]
LNS in females
[edit]While carrier females are generally anasymptomatic condition,they do experience an increase in uric acid excretion, and some may develop symptoms ofhyperuricemia,and experience gout in their later years. Testing in this context has no clinical consequence, but it may reveal the possibility of transmitting the trait to male children. Women may also require testing if a male child develops LNS. In this instance, a negative test means the son's disease is the result of a new mutation, and the risk in siblings is not increased.[17]
Females who carry one copy of the defective gene are carriers with a 50% chance of passing the disease on to their sons. In order for a female to be affected, she would need to have two copies of the mutated gene, one of which would be inherited from her father. Males affected with LNS do not usually have children due to the debilitating effects of the disease. It is possible for a female to inherit an X chromosome from her unaffected father, who carries a new mutation of the HGPRT gene. Under these circumstances, a girl could be born with LNS, and though there are a few reports of this happening, it is very rare. The overwhelming majority of patients with LNS are male.[citation needed]
Less severe forms
[edit]A less severe, related disease, partial HPRT deficiency, is known as Kelley–Seegmiller syndrome (Lesch–Nyhan syndrome involves total HPRT deficiency). Symptoms generally involve less neurological involvement but the disease still causes gout and kidney stones.[18]
Genetics
[edit]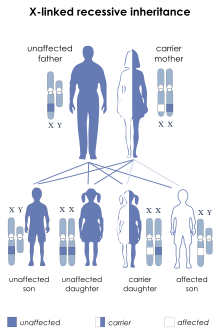
LNS is due tomutationsin theHPRT1gene,[3][19]so named because it codes for theenzymehypoxanthine-guanine phosphoribosyltransferase(HPRT or HGPRT,EC2.4.2.8). This enzyme is involved in the biochemical pathways the body uses to producepurines,one of the components ofDNAandRNA.Defects of this enzyme lead to increased production ofuric acid.Since theHPRTgene is located on theX chromosome,LNS is anX-linkedinherited disease.[citation needed]
The father of an affected male will not be the carrier of the mutantallele,and will not have the disease. Anobligate carrierwould be a woman who has an affected son and one other affected relative in the maternal line.[citation needed]
If a woman is the first in her family with an affected son,Haldane's rulepredicts a 2/3 chance that she is a carrier and a 1/3 chance that the son has a newgermline mutation.[citation needed]
The risk to siblings of an affected individual depends upon the carrier status of the mother herself. A 50% chance is given to any female who is a carrier to transmit the HPRT1 mutation in eachpregnancy.Sons who inherit the mutation will be affected while daughters who inherit the mutation are carriers. Therefore, with each pregnancy, a carrier female has a 25% chance of having a male that is affected, a 25% chance of having a female that is a carrier, and a 50% chance of having a normal male or female.[citation needed]
Males with LNS do not reproduce due to the characteristics of the disease. However, if a male with a less severephenotypereproduces, all of his daughters are carriers, and none of his sons will be affected.[citation needed]
Pathophysiology
[edit]
As in otherX-linkeddiseases, males are affected because they only have one copy of the X chromosome. In Lesch–Nyhan syndrome, the defective gene is that forhypoxanthine-guanine phosphoribosyltransferase(HGPRT), a participant in the 'recycling' ofpurinenucleotides.Female carriers have a second X chromosome, which contains a "normal" copy of HPRT, preventing the disease from developing, though they may have increased risk of hyperuricemia.[citation needed]
A large number of mutations of HPRT are known. Mutations that only mildly decrease the enzyme's function do not normally cause the severe form of LNS, but do produce a milder form of the disease which still features purine overproduction accompanied by susceptibility togoutanduric acidnephrolithiasis.[citation needed]
Formation of DNA (duringcell division) requiresnucleotides,molecules that are the building blocks for DNA. The purine bases (adenineandguanine) andpyrimidinebases (thymineandcytosine) are bound to deoxyribose and phosphate and incorporated as necessary. Normally, the nucleotides are synthesizedde novofromamino acidsand other precursors. A small part, however, is 'recycled' from degraded DNA of broken-down cells. This is termed the "salvage pathway".[citation needed]
HGPRT is the "salvage enzyme" for the purines: it channelshypoxanthineandguanineback into DNA synthesis. Failure of this enzyme has two results:[citation needed]
- Cell breakdown products cannot be reused, and are therefore degraded. This gives rise to increaseduric acid,a purine breakdown product.
- Thede novopathway is stimulated due to an excess of PRPP (5-phospho-D-ribosyl-1-pyrophosphate or simply phosphoribosyl-pyrophosphate).
It was previously unclear whether the neurological abnormalities in LNS were due to uric acidneurotoxicityor to a relative shortage in "new" purinenucleotidesduring essential synthesis steps. Genetic mutations affecting the enzymes of thede novosynthesis pathway may possibly contribute to the disease, although these are rare or unknown. Uric acid has been suggested as a possible cause of neurotoxicity but this is unproven.[citation needed]
Importantly, evidence suggests that one or more lesions instriataldopaminergicpathways may be central to the neurological deficits, especially the choreoathetoid dyskinesia and self-mutilation.[20][21][22]6-hydroxydopaminetoxicity in rodents may be a useful animal model for the syndrome, although this is not proven.[23] However, the link between dopamine and purine synthesis is a nucleotide calledguanosine triphosphateor 'GTP'. The first step of dopamine synthesis isGTP cyclohydrolase,and significantly a deficiency of this step produces a syndrome that has a neuropathology similar to LNS. Thus a lack of HGPRT may produce anucleotide deficiency(specifically: GTP deficiency) disorder, resulting in dopamine deficiency.[24]
Another animal model for LNS has been proposed to arise from oxidative damage, caused by thehyperuricemiaaccompanying LNS. This is based on the theory thaturic acidis a powerfulreducing agentand likely an important humanantioxidant,in high concentration in blood. Thus, it has been suggested thatfree radicals,oxidative stress,andreactive oxygen speciesmay play some role in the neuropathology of LNS.[22][25][26][non-primary source needed]
However, some evidence suggests against a role for uric acid in the neuropathology of Lesch–Nyhan syndrome:
- Hyperuricemia associated with classicprimary gout,which is caused by low uric acidrenal clearancerather than uric acid overproduction, is not associated with neuropathology.
- Hypouricemia occurs in a number of purine disorders, in particularxanthinuria.Despite having complete absence of blood uric acid, xanthinuria patients do not have any neuropathology, nor any other disease states – other than the kidney stones caused by accumulation of insoluble xanthine in lieu of uric acid.[27]
Similarly, uric acid does not penetrate the blood–brain barrier well. However, oxidative stress due to uric acid is now thought to figure inmetabolic syndrome,atherosclerosis,andstroke,all syndromes associated with high uric acid levels. Similarly,Superoxide dismutase( "SOD" ) andSOD-mimeticssuch asTEMPOLameliorate the effects of hyperuricemia. Likewise, 6-hydroxydopamine (the putative animal model for Lesch–Nyhan's neuropathy) apparently acts as aneurotoxinby generation of reactive oxygen species. It may be that oxidative stress induced by some other oxypurine such as xanthine causes the disease.[citation needed]
Diagnosis
[edit]When an affected individual has fully developed the three clinical elements of uric acid overproduction, neurologic dysfunction, and cognitive and behavioral disturbances, diagnosis of LNS is easily made. Diagnosis is less easy in the early stages, when the three features are not yet obvious. Signs of self-injurious behavior (SIB), results of pedigree analysis and novel molecular biology withgenetic testing(called as Diagnostic triad for LNS), often confirms the diagnosis.[28]Suspicion often comes about when the developmental delay of the individual is associated with hyperuricemia. Otherwise, the diagnosis should be alleged when developmental delay is associated with kidney stones (nephrolithiasis) or blood in the urine (hematuria), caused by uric acid stones. For the most part, Lesch–Nyhan syndrome is first suspected when self-inflicted injury behavior develops. However, self-injurious behaviors occur in other conditions, including nonspecificintellectual disability,autism,Rett syndrome,Cornelia de Lange syndrome,Tourette syndrome,familial dysautonomia,choreoacanthocytosis,sensory neuropathyincluding hereditary sensory neuropathy type 1, and several psychiatric conditions. Of these, only individuals with Lesch–Nyhan syndrome, de Lange syndrome, and familial dysautonomia recurrently display loss of tissue as a consequence. Biting the fingers and lips is a definitive feature of Lesch–Nyhan syndrome; in other syndromes associated with self-injury, the behaviors usually consist of head banging and nonspecific self-mutilation, but not biting of the cheeks, lips and fingers. Lesch–Nyhan syndrome ought to be clearly considered only when self-injurious behavior takes place in conjunction with hyperuricemia and neurological dysfunction.[29]
Diagnostic approach
[edit]The urate tocreatinine(breakdown product of creatine phosphate in muscle) concentration ratio in urine is elevated. This is a good indicator of acid overproduction. For children under ten years of age with LNS, a urate to creatinine ratio above two is typically found. Twenty-four-hour urate excretion of more than 20 mg/kg is also typical but is notdiagnostic.Hyperuricemia (serum uric acid concentration of >8 mg/dL) is often present but not reliable enough for diagnosis. Activity of the HGPRT enzyme incellsfrom any type oftissue(e.g.,blood,culturedfibroblasts,orlymphoblasts) that is less than 1.5% of normal enzyme activity confirms the diagnosis of Lesch–Nyhan syndrome. Molecular genetic studies of the HPRT gene mutations may confirm diagnosis, and are particularly helpful for subsequent 'carrier testing' in at-risk females such as close family relatives on the female side.[citation needed]
Testing
[edit]The use ofbiochemicaltesting for the detection of carriers is technically demanding and not often used. Biochemical analyses that have been performed on hair bulbs from at risk women have had a small number of bothfalse positiveand false negative outcomes. If only a suspected carrier female is available for mutation testing, it may be appropriate to grow herlymphocytesin 6-thioguanine (apurine analogue), which allows only HGPRT-deficient cells to survive. A mutant frequency of 0.5–5.0 × 10−2is found in carrier females, while a non-carrier female has a frequency of 1–20 × 10−6.This frequency is usually diagnostic by itself.[citation needed]
Molecular genetic testing is the most effective method of testing, as HPRT1 is the only gene known to be associated with LNS. Individuals who display the full Lesch–Nyhanphenotypeall have mutations in the HPRT1 gene. Sequence analysis ofmRNAis available clinically and can be utilized in order to detect HPRT1 mutations in males affected with Lesch–Nyhan syndrome. Techniques such as RT-PCR,multiplex genomic PCR,and sequence analysis (cDNA and genomic DNA), used for the diagnosis of genetic diseases, are performed on a research basis. If RT-PCR tests result in cDNA showing the absence of an entireexonor exons, then multiplex genomic PCR testing is performed. Multiplex genomic PCR testing amplifies the nine exons of the HPRT1 gene as eight PCR products. If the exon in question is deleted, the corresponding band will be missing from the multiplex PCR. However, if the exon is present, the exon is sequenced to identify the mutation, therefore causing exclusion of the exon from cDNA. If no cDNA is created by RT-PCR, then multiplex PCR is performed on the notion that most or all of the gene is obliterated.[citation needed]
Treatment
[edit]Treatment for LNS is symptomatic. Gout can be treated withallopurinolto control excessive amounts ofuric acid.Kidney stones may be treated withlithotripsy,a technique for breaking up kidney stones using shock waves or laser beams. There is no standard treatment for the neurological symptoms of LNS. Some may be relieved with the drugscarbidopa/levodopa,diazepam,phenobarbital,orhaloperidol.[5]
It is essential that the overproduction of uric acid be controlled in order to reduce the risk ofnephropathy,nephrolithiasis,and gouty arthritis. The drugallopurinolis utilized to stop the conversion ofoxypurinesinto uric acid, and prevent the development of subsequentarthritic tophi(produced after having chronic gout),kidney stones,andnephropathy,the resulting kidney disease. Allopurinol is taken orally, at a typical dose of 3–20 mg/kg per day. The dose is then adjusted to bring the uric acid level down into the normal range (<3 mg/dL). Most affected individuals can be treated with allopurinol all through life.[citation needed]
No medication is effective in controlling theextrapyramidalmotor features of the disease.Spasticity,however, can be reduced by the administration ofbaclofenorbenzodiazepines.[citation needed]
There has previously been no effective method of treatment for the neurobehavioral aspects of the disease. Even children treated from birth with allopurinol develop behavioral and neurologic problems, despite never having had high serum concentrations of uric acid. Self-injurious and other behaviors are best managed by a combination of medical, physical, and behavioral interventions. The self-mutilation is often reduced by using restraints. Sixty percent of individuals have their teeth extracted[citation needed]in order to avoid self-injury, which families have found to be an effective management technique.[citation needed]Because stress increases self-injury, behavioral management through aversive techniques (which would normally reduce self-injury) actually increases self-injury in individuals with LNS. Nearly all affected individuals need restraints to prevent self-injury, and are restrained more than 75% of the time. This is often at their own request, and occasionally involves restraints that would appear to be ineffective, as they do not physically prevent biting. Families report that affected individuals are more at ease when restrained.[citation needed]
The Matheny Medical and Educational CenterMatheny | A Non-profit Organization for People with Special Needsin Peapack, NJ, has[when?]six Lesch–Nyhan syndrome patients, believed to be the largest concentration of LNS cases in one location, and is recognized as the leading source of information on care issues.
Treatment for LNS patients, according to Gary E. Eddey, MD, medical director[clarification needed],should include: 1) Judicious use of protective devices; 2) Utilization of a behavioral technique commonly referred to as 'selective ignoring' with redirection of activities; and 3) Occasional use of medications.[citation needed]
An article in the August 13, 2007 issue ofThe New Yorkermagazine, written byRichard Preston,discusses "deep-brain stimulation"as a possible treatment. It has been performed on a few patients with Lesch–Nyhan syndrome by Dr. Takaomi Taira in Tokyo and by a group in France led by Dr. Philippe Coubes. Some patients experienced a decrease in spastic self-injurious symptoms. The technique was developed for treating people withParkinson's disease,according to Preston, over 20 years ago. The treatment involves invasive surgery to place wires that carry a continuous electric current into a specific region of the brain.[30]
An encouraging advance in the treatment of the neurobehavioural aspects of LNS was the publication in the October, 2006 issue ofJournal of Inherited Metabolic Diseaseof an experimental therapy giving oralS-adenosyl-methionine(SAMe).[31] This drug is anucleotideprecursorthat provides a readily absorbed purine, which is known to be transported across theblood–brain barrier.Administration of SAMe to adult LNS patients was shown to provide improvement in neurobehavioural and other neurological attributes. The drug is available without prescription and has been widely used fordepression,but its use for treating LNS should be undertaken only under strict medical supervision, as side effects are known.[citation needed]
Prognosis
[edit]The prognosis for individuals with severe LNS is poor. Death is usually due tokidney failureor complications from hypotonia, in the first or second decade of life.Less severe formshave better prognosis.[5]
History
[edit]Michael Leschwas a medical student atJohns HopkinsandWilliam Nyhan,apediatricianand biochemical geneticist, was his mentor when the two identified LNS and its associated hyperuricemia in two affected brothers, ages 4 and 8.[32]Lesch and Nyhan published their findings in 1964.[33]Within three years, the metabolic cause was identified byJ. Edwin Seegmillerand his colleagues at theNIH.[34]
References
[edit]- ^James, William D.; Berger, Timothy G.; et al. (2006).Andrews' Diseases of the Skin: clinical Dermatology.Saunders Elsevier. p. 546.ISBN978-0-7216-2921-6.
- ^ab"Lesch Nyhan Syndrome".Retrieved24 February2022.
- ^abLesch–Nyhan syndrome.Genetics Home Reference. Retrieved on 2007-05-24.
- ^Ole Daniel Enersen.Lesch-Nyhan syndromeatWho Named It?
- ^abcLesch-Nyhan SyndromeatNINDS
- ^Hladnik U, Nyhan WL, Bertelli M (September 2008)."Variable expression of HPRT deficiency in 5 members of a family with the same mutation".Arch. Neurol.65(9): 1240–3.doi:10.1001/archneur.65.9.1240.PMID18779430.
- ^Cakmakli, H. F.; Torres, R. J.; Menendez, A.; Yalcin-Cakmakli, G.; Porter, C. C.; Puig, J. G.; Jinnah, H. A. (2019)."Macrocytic anemia in Lesch-Nyhan disease and its variants".Genetics in Medicine.21(2): 353–360.doi:10.1038/s41436-018-0053-1.PMC6281870.PMID29875418.
- ^Cakmakli, H. F.; Torres, R. J.; Menendez, A.; Yalcin-Cakmakli, G.; Porter, C. C.; Puig, J. G.; Jinnah, H. A. (2018)."Macrocytic Anemia in Lesch-Nyhan Disease and its Variants".Genetics in Medicine.21(2): 353–360.doi:10.1038/s41436-018-0053-1.PMC6281870.PMID29875418.
- ^Nagao, T.; Hirokawa, M. (2017)."Diagnosis and treatment of macrocytic anemias in adults".Journal of General and Family Medicine.18(5): 200–204.doi:10.1002/jgf2.31.PMC5689413.PMID29264027.
- ^"Lesch Nyhan Syndrome".NORD (National Organization for Rare Disorders).Retrieved3 January2023.
- ^Nanagiri, Apoorva; Shabbir, Nadeem (2022)."Lesch Nyhan Syndrome".StatPearls.StatPearls Publishing.PMID32310539.Retrieved3 January2023.
- ^"Delineation of the motor disorder of Lesch–Nyhan disease".academic.oup.Brain.Retrieved3 January2023.
- ^"SSA - POMS: DI 23022.220 - Lesch-Nyhan Syndrome (LNS) - 08/28/2020".secure.ssa.gov.Retrieved3 January2023.
- ^Cauwels RG, Martens LC (2005)."Self-mutilation behaviour in Lesch–Nyhan syndrome".Journal of Oral Pathology and Medicine.34(9): 573–5.doi:10.1111/j.1600-0714.2005.00330.x.PMID16138897.
- ^abGualtieri, C. Thomas (2002).Brain Injury and Mental Retardation: Psychopharmacology and Neuropsychiatry,p. 257. Lippincott Williams & Wilkins.ISBN0-7817-3473-8.[1]
- ^"Attenuated variants of Lesch-Nyhan disease".academic.oup.Retrieved3 January2023.
- ^Puig; Mateos; Torres; Buño (1998). "Purine metabolism in female heterozygotes for hypoxanthine-guanine phosphoribosyltransferase deficiency".European Journal of Clinical Investigation.28(11). Wiley: 950–957.doi:10.1046/j.1365-2362.1998.00392.x.ISSN0014-2972.PMID9824441.S2CID23770074.
- ^Augoustides-Savvopoulou P, Papachristou F, Fairbanks LD, Dimitrakopoulos K, Marinaki AM, Simmonds HA (2002)."Partial hypoxanthine-Guanine phosphoribosyltransferase deficiency as the unsuspected cause of renal disease spanning three generations: a cautionary tale".Pediatrics.109(1): E17.doi:10.1542/peds.109.1.e17.PMID11773585.
- ^Lesch–Nyhan syndrome.NCBI Genes and disease. Retrieved on 2007-04-12
- ^Proctor, P (26 December 1970). "Levodopa Side-effects and the Lesch-Nyhan Syndrome".Lancet.296(7687): 1367.doi:10.1016/S0140-6736(70)92399-8.PMID4098945.
- ^Nyhan WL (2000)."Dopamine function in Lesch–Nyhan disease".Environ. Health Perspect.108(Suppl 3): 409–11.doi:10.2307/3454529.JSTOR3454529.PMC1637829.PMID10852837.
- ^abVisser J, Smith D, Moy S, Breese G, Friedmann T, Rothstein J, Jinnah H (2002). "Oxidative stress and dopamine deficiency in a genetic mouse model of Lesch–Nyhan disease".Brain Res Dev Brain Res.133(2): 127–39.doi:10.1016/S0165-3806(02)00280-8.PMID11882343.
- ^Breese GR, Knapp DJ, Criswell HE, Moy SS, Papadeas ST, Blake BL (2005). "The neonate-6-hydroxydopamine-lesioned rat: a model for clinical neuroscience and neurobiological principles".Brain Res. Brain Res. Rev.48(1): 57–73.doi:10.1016/j.brainresrev.2004.08.004.PMID15708628.S2CID22599841.
- ^Deutsch SI; Long KD; Rosse RB; Mastropaolo J; Eller J. (Jan–Feb 2005). "Hypothesized deficiency of guanine-based purines may contribute to abnormalities of neurodevelopment, neuromodulation, and neurotransmission in Lesch–Nyhan syndrome".Clin. Neuropharmacol.28(1): 28–37.doi:10.1097/01.wnf.0000152043.36198.25.PMID15711436.S2CID36457793.
- ^Saugstad O, Marklund S (1988). "High activities of erythrocyte glutathione peroxidase in patients with the Lesch–Nyhan syndrome".Acta Med Scand.224(3): 281–5.doi:10.1111/j.0954-6820.1988.tb19374.x.PMID3239456.
- ^Bavaresco C, Chiarani F, Matté C, Wajner M, Netto C, de Souza Wyse A (2005). "Effect of hypoxanthine on Na+,K+-ATPase activity and some parameters of oxidative stress in rat striatum".Brain Res.1041(2): 198–204.doi:10.1016/j.brainres.2005.02.012.PMID15829228.S2CID22575382.
- ^Kudo M; Moteki T; Sasaki T; Konno Y; Ujiie S; Onose A; Mizugaki M; Ishikawa M; Hiratsuka M. (March 2008). "Functional characterization of human xanthine oxidase allelic variants".Pharmacogenet Genomics.18(3): 243–51.doi:10.1097/FPC.0b013e3282f55e2e.PMID18300946.S2CID8140455.
- ^Tewari, Nitesh; Mathur, Vijay Prakash; Sardana, Divesh; Bansal, Kalpana (2017)."Lesch-Nyhan syndrome: The saga of metabolic abnormalities and self-injurious behavior".Intractable & Rare Diseases Research.6(1): 65–68.doi:10.5582/irdr.2016.01076.ISSN2186-361X.PMC5359358.PMID28357186.
- ^Tewari N, Mathur VP, Sardana D, Bansal K (February 2017)."Lesch-Nyhan syndrome: The saga of metabolic abnormalities and self-injurious behavior".Intractable Rare Dis Res.6(1): 65–68.doi:10.5582/irdr.2016.01076.PMC5359358.PMID28357186.
- ^Preston, Richard(August 2007)."An Error in the Code".The New Yorker.p. 30. Archived fromthe originalon 2012-09-05.Retrieved2008-09-14.
- ^Glick N (October 2006). "Dramatic reduction in self-injury in Lesch–Nyhan disease following S-adenosylmethionine administration".J. Inherit. Metab. Dis.29(5): 687.doi:10.1007/s10545-006-0229-8.PMID16906475.S2CID33099025.
- ^Nyhan WL (1997)."The recognition of Lesch–Nyhan syndrome as an inborn error of purine metabolism"(PDF).J. Inherit. Metab. Dis.20(2): 171–8.doi:10.1023/A:1005348504512.PMID9211189.S2CID37373603.[permanent dead link]
- ^Lesch M, Nyhan WL (1964). "A familial disorder of uric acid metabolism and central nervous system function".Am. J. Med.36(4): 561–70.doi:10.1016/0002-9343(64)90104-4.PMID14142409.
- ^Seegmiller JE, Rosenbloom FM, Kelley WN (1967). "Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis".Science.155(770): 1682–4.Bibcode:1967Sci...155.1682S.doi:10.1126/science.155.3770.1682.PMID6020292.S2CID45609754.
