Lomevactone
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChemCID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C18H17ClO2 |
| Molar mass | 300.78g·mol−1 |
| 3D model (JSmol) | |
| |
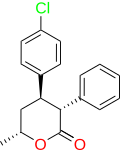
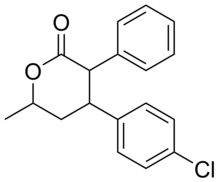
Lomevactone(INN;developmental code nameDR-250) is adrugdescribed as apsychostimulantandantidepressantwhich wassynthesizedand assayed in the 1980s, but was never marketed.[1][2]
Stereoisomers
[edit]There are eight possiblestereoisomersof lomevactone. It is the (3R,4R,6R)-form that has the psychotherapeutic properties.[3][4]
Synthesis
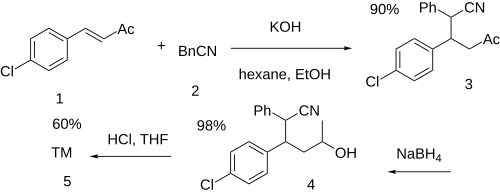
[edit]The conjugate 1,4-alkylation reaction between 4-chlorobenzylideneacetone (1) andphenylacetonitrile(2) gives 3-(4-chlorophenyl)-5-oxo-2-phenylhexanenitrile (3). The selective reduction of the keto group to the alcohol withsodium borohydridegives 3-(4-chlorophenyl)-5-hydroxy-2-phenylhexanenitrile (4). Hydrolysis of the nitrile to an acid gives 3-(4-chlorophenyl)-5-hydroxy-2-phenylhexanoic acid. This is followed by lactone formation completing the synthesis of lomevactone (5).
References
[edit]- ^David J. Triggle (1997).Dictionary of pharmacological agents.London: Chapman & Hall.ISBN0-412-46630-9.
- ^Poncelet M, Chermat R, Soubrie P, Simon P (1983). "The progressive ratio schedule as a model for studying the psychomotor stimulant activity of drugs in the rat".Psychopharmacology.80(2): 184–9.doi:10.1007/BF00427967.PMID6136063.S2CID2372145.
- ^Axiotis, S.; Druex, J.; Perrin, M.; Royer, J. (1982). "Conformations in the tetrahydropyran-2-one ring". Tetrahedron. 38 (4): 499–504. doi:10.1016/0040-4020(82)80093-8.
- ^Axiotis, S., Sollier, J.-C., Dreux, J., Chermat, R., Poncelet, M., Simon, P. (July 1987). "Tétrahydropyrones-2 III. Recherche d'une activité psychostimulante spécifique".European Journal of Medicinal Chemistry.22(4): 293–303.doi:10.1016/0223-5234(87)90266-2.
- ^Axiotis, S. et al, Eur. J. Med. Chem.-Chim. Ther., 1981, 16, 431, 439.
- ^Pierre Simon & Jacques Dreux,U.S. patent 4,287,206(1981 to Sanofi Aventis France).
- ^시몽 삐에르 & 드로 짝끄, KR830002288 (1983).