MERRF syndrome
| MERRF syndrome | |
|---|---|
| Other names | Fukuhara syndrome |
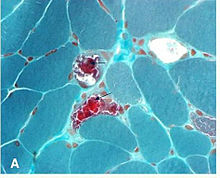 | |
| "ragged red fibers" in MERRF syndrome | |
| Specialty | Neurology |
MERRF syndrome(ormyoclonic epilepsy with ragged red fibers) is amitochondrial disease.It is extremely rare, and has varying degrees ofexpressivityowing toheteroplasmy.[1]MERRF syndrome affects different parts of the body, particularly themusclesandnervous system.[2]The signs and symptoms of this disorder appear at an early age, generallychildhoodoradolescence.The causes of MERRF syndrome are difficult to determine, but because it is a mitochondrial disorder, it can be caused by the mutation ofnuclear DNAormitochondrial DNA.[3]The classification of this disease varies from patient to patient, since many individuals do not fall into one specific disease category. The primary features displayed on a person with MERRF includemyoclonus,seizures,cerebellar ataxia,myopathy,[3]and ragged red fibers (RRF) on muscle biopsy, leading to the disease's name. Secondary features includedementia,opticatrophy,bilateral deafness,peripheral neuropathy,spasticity,ormultiple lipomata.Mitochondrial disorders, including MERRFS, may present at any age.[4]
Symptoms and signs
[edit]An individual displaying MERRFs syndrome will manifest not only a single symptom, but patients regularly display more than one affected body part at a time. It has been observed that patients with MERRF syndrome will primarily displaymyoclonusas a first symptom. There may also beseizures,cerebellar ataxiaandmyopathy.[3]Secondary features can includedementia,opticatrophy,bilateral deafness,peripheral neuropathy,spasticity,multiple lipomata,and/or cardiomyopathy withWolff Parkinson-White syndrome.Most patients will not exhibit all of these symptoms, but more than one of these symptoms will be present in a patient who has been diagnosed with MERRF disease. Mitochondrial disorders, including MERRF, may present at any age.[4]Due to the multiple symptoms presented by the individual, the severity of the syndrome is very difficult to evaluate.[5]
Causes
[edit]
The cause of MERRF disorder is due tomutationsin themitochondrialgenome.This means that it is a pathological variant inmtDNA(mitochondrial DNA) and is transmitted bymaternalinheritance.Fourpoint mutationsin the genome can be identified that are associated with MERRF: m.A8344G, m.T8356C, m.G8361A, and m.G8363A. The point mutation m.A8344G is most commonly associated with MERRF,[6]in a study published by Paul Jose Lorenzoni from the Department of neurology atUniversity of Panama[7]stated that 80% of the patients with MERRF disease exhibited this point mutation. This point mutation disrupts the mitochondrial gene fortRNA-Lys. This disrupts the synthesis of proteins. The remaining mutations only account for 10% of cases, and the remaining 10% of the patients with MERRF did not have an identifiable mutation in themitochondrial DNA.[citation needed]
Manygenesare involved.[8]These genes include:
It involves the following characteristics:
- progressive myoclonic epilepsy
- "Ragged Red Fibers"- clumps of diseasedmitochondriaaccumulate in the subsarcolemmal region of the muscle fiber and appear as "Ragged Red Fibers" when muscle is stained with modifiedGömöri trichrome stain.
There is currently no cure for MERRF.[citation needed]
Mechanism
[edit]The mechanism by which MERRFs syndrome occur is not yet well understood. The human mitochondrialtRNAmutationsare associated with a variety ofdiseasesincluding mitochondrialmyopathies.[12]However, it is understood that defects in themitochondrial DNA(mtDNA) have been associated with these diseases, and studies have been able to assign biochemical defects.[13]One of these defects has to do with the decreasedenergyavailable forcellprocesses. Asmusclesare stained withGömöri trichrome,characteristicragged red fibersare visible under themicroscope.This appearance is due to the accumulation of abnormal mitochondria below theplasma membraneof themuscle fiber.[6]These may extend throughout the muscle fiber as the disease severity increases. The mitochondrial aggregates cause the contour of the muscle fiber to become irregular, leading to the "ragged" appearance.[3]
Diagnosis
[edit]Thediagnosisvaries from individual to individual. Each is evaluated and diagnosed according to age, clinicalphenotype,and pressed inheritance pattern.[14]If the individual has been experiencing myoclonus, thedoctorwill run a series of genetic studies to determine if it is a mitochondrial disorder.[citation needed]
The moleculargenetic studiesare run to identify the reason of for the mutations underlying the mitochondrial dysfunction. This approach will avoid the need for a muscle biopsy or an exhaustive metabolic evaluation. Aftersequencingthemitochondrial genomes,fourpoints mutationsin thegenomecan be identified which are associated with MERRF: A8344G, T8356C, G8361A, and G8363A. The point mutation[9]A8344G is mostly associated with MERRF,[6]in a study published byPaul Jose Lorenzonifrom the Department of neurology atUniversity of Panama[7]stated that 80% of the patients with MERRF disease exhibited this point mutation. The remaining mutations only account for 10% of cases, and the remaining 10% of the patients with MERRF did not have an identifiable mutation in themitochondrial DNA.[12]
If a patient does not exhibit mitochondrial DNA mutations, there are other ways that they can be diagnosed with MERRF. They can go throughcomputed tomography(CT) ormagnetic resonance imaging(MRI).The classification for the severity of MERRF syndrome is difficult to distinguish since most individuals will exhibit multi-symptoms.[12]This is often necessary for children with complex neurologic or multi-system involvement, as described below.[4]
History and physical examination of the patient
[edit]A detailed family history should be obtained from at least three generations, particularly if there have been anyneonatalandchildhooddeaths. A family history may also indicate if any family members exhibit features of the multi-system disease, specifically if there has been maternal inheritance. This would show transmission of the disease only to females, or if there is a family member who experienced a multi-system involvement such as:[14]braincondition that a family member has been record to have such asseizures,dystonia,ataxia,or stroke-like episodes. There may also be optic atrophy,skeletal musclewith a history ofmyalgia,weakness, orptosis.Family history may also includeneuropathyanddysautonomia,orheartconditions such ascardiomyopathy.The patient's history might also exhibitkidneyproblems, such as proximalnephrondysfunction. There may also beendocrineconditions, such asdiabetesorhypoparathyroidism.The patient might have also had agastrointestinalcondition which could have been due toliver disease,as well as episodes of nausea or vomiting. Multiplelipomasin theskin,sideroblastic anemia andpancytopeniain themetabolicsystem, or short stature might all be examples of patients with possible symptoms of MERRF disease.[citation needed]
Treatment
[edit]Like manymitochondrial diseases,there is no cure for MERRF, no matter the means for diagnosis of the disease. The treatment is primarilysymptomatic.High doses ofcoenzyme Q10,B complex vitamins, andL-Carnitineare used for the alteredmetabolicprocessing that results in the disease.[9]There is very little success with these treatments astherapiesin hopes of improving mitochondrial function.[15]The treatment only alleviates symptoms, and these do not prevent the disease from progressing. Patients withconcomitant disease,such asdiabetes,deafness,or cardiacdisease,are treated in combination to manage symptoms.[citation needed]
Research
[edit]TheJournal of Child Neurologypublished a paper in 2011 that discusses possible new methods to test for MERRF and othermitochondrial diseasesthrough a simple swabbing technique. This is a less invasive technique which allows for an analysis ofbuccalmitochondrial DNA,and showed significant amounts of the common 5 kb and 7.4 kb mitochondrial DNA deletions, which are also detectable inblood.[16]This study suggests that a buccal swab approach can be used to informatively examine mitochondrial dysfunction in children with seizures and may be applicable to screening mitochondrial disease with other clinical presentations.[citation needed]
TheProceedings of theNational Academy of Science of the United States of Americapublished an article in 2007 investigating the human mitochondrial tRNA (hmt-tRNA) mutations which are associated withmitochondrial myopathies.Since the current understanding of the precisemolecular mechanismsof these mutations is limited, there is no efficient method to treat their associated mitochondrial diseases. All pathogenic mutants displayedpleiotropicphenotypes,with the exception of the G34Aanticodonmutation,which solely affectedaminoacylation.[12]
Society and culture
[edit]MERRF syndrome was the final diagnosis of seventh episode of third season on the showHouse, M.D..
See also
[edit]References
[edit]- ^Gene Reviews: MERRF
- ^DiMauro, Salvatore;Hirano, Michio (1993)."MERRF".In Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E.; Bean, Lora J.H.; Mefford, Heather C.; Stephens, Karen; Amemiya, Anne; Ledbetter, Nikki (eds.).GeneReviews.Seattle (WA): University of Washington, Seattle.PMID20301693.
- ^abcdChinnery, Patrick F. (1993)."Mitochondrial Disorders Overview".In Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E.; Bean, Lora J.H.; Mefford, Heather C.; Stephens, Karen; Amemiya, Anne; Ledbetter, Nikki (eds.).GeneReviews.Seattle (WA): University of Washington, Seattle.PMID20301403.
- ^abc"Mitochondrial myopathies: Clinical features and diagnosis".uptodate.Retrieved2017-11-07.
- ^abMelone MA, Tessa A, Petrini S, et al. (February 2004). "Revelation of a new mitochondrial DNA mutation (G12147A) in a MELAS/MERFF phenotype".Arch. Neurol.61(2): 269–72.doi:10.1001/archneur.61.2.269.PMID14967777.S2CID9418186.
- ^abc"Myoclonus Epilepsy Associated with Ragged-Red Fibers (MERRF) Diagnosis Discussed by Researchers - Mitochondrial Disease News".Mitochondrial Disease News.2015-05-04.Retrieved2017-11-08.
- ^abLorenzoni, Paulo José; Scola, Rosana Herminia; Kay, Cláudia Suemi Kamoi; Silvado, Carlos Eduardo S.; Werneck, Lineu Cesar; Lorenzoni, Paulo José; Scola, Rosana Herminia; Kay, Cláudia Suemi Kamoi; Silvado, Carlos Eduardo S. (October 2014)."When should MERRF (myoclonus epilepsy associated with ragged-red fibers) be the diagnosis?".Arquivos de Neuro-Psiquiatria.72(10): 803–811.doi:10.1590/0004-282x20140124.ISSN0004-282X.PMID25337734.
- ^Online Mendelian Inheritance in Man(OMIM):MYOCLONIC EPILEPSY ASSOCIATED WITH RAGGED-RED FIBERS; MERRF - 545000
- ^abcZeviani M, Muntoni F, Savarese N, et al. (1993). "A MERRF/MELAS overlap syndrome associated with a new point mutation in the mitochondrial DNA tRNA(Lys) gene".Eur. J. Hum. Genet.1(1): 80–7.doi:10.1159/000472390.PMID8069654.S2CID22766360.
- ^Nakamura M, Nakano S, Goto Y, et al. (September 1995). "A novel point mutation in the mitochondrial tRNA(Ser(UCN)) gene detected in a family with MERRF/MELAS overlap syndrome".Biochem. Biophys. Res. Commun.214(1): 86–93.doi:10.1006/bbrc.1995.2260.PMID7669057.
- ^Mancuso M, Filosto M, Mootha VK, et al. (June 2004). "A novel mitochondrial tRNAPhe mutation causes MERRF syndrome".Neurology.62(11): 2119–21.doi:10.1212/01.wnl.0000127608.48406.f1.PMID15184630.S2CID12423569.
- ^abcdLing, Jiqiang; Roy, Hervé; Qin, Daoming; Rubio, Mary Anne T.; Alfonzo, Juan D.; Fredrick, Kurt; Ibba, Michael (2007-09-25)."Pathogenic mechanism of a human mitochondrial tRNAPhe mutation associated with myoclonic epilepsy with ragged red fibers syndrome".Proceedings of the National Academy of Sciences of the United States of America.104(39): 15299–15304.Bibcode:2007PNAS..10415299L.doi:10.1073/pnas.0704441104.ISSN0027-8424.PMC2000536.PMID17878308.
- ^McKenzie, Matthew; Liolitsa, Danae; Hanna, Michael G. (2004-03-01). "Mitochondrial Disease: Mutations and Mechanisms".Neurochemical Research.29(3): 589–600.doi:10.1023/B:NERE.0000014829.42364.dd.ISSN0364-3190.PMID15038606.S2CID12265373.
- ^ab"Mitochondrial myopathies: Clinical features and diagnosis".uptodate.Retrieved2017-11-08.
- ^Gene reviews: MERRF: Management of patients
- ^Yorns, William R.; Valencia, Ignacio; Jayaraman, Aditya; Sheth, Sudip; Legido, Agustin; Goldenthal, Michael J. (2011-11-22). "Buccal Swab Analysis of Mitochondrial Enzyme Deficiency and DNA Defects in a Child With Suspected Myoclonic Epilepsy and Ragged Red Fibers (MERRF)".Journal of Child Neurology.27(3): 398–401.doi:10.1177/0883073811420870.PMID22114216.S2CID23912193.
External links
[edit]- MERRF+Syndromeat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- merrfatNIH/UWGeneTests
