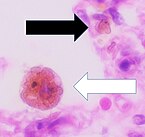
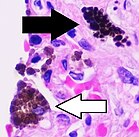
Macrophage
| Macrophage | |
|---|---|
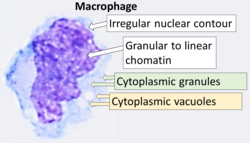 Cytology of a macrophage with typical features.Wright stain. | |
| Details | |
| Pronunciation | /ˈmakrə(ʊ)feɪdʒ/ |
| System | Immune system |
| Function | Phagocytosis |
| Identifiers | |
| Latin | macrophagocytus |
| Acronym(s) | Mφ,MΦ |
| MeSH | D008264 |
| TH | H2.00.03.0.01007 |
| FMA | 63261 |
| Anatomical terms of microanatomy | |
Macrophages(/ˈmækroʊfeɪdʒ/;abbreviatedMφ,MΦorMP) are a type ofwhite blood cellof theinnate immune systemthat engulf and digest pathogens, such ascancer cells,microbes,cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface.[1][2]This process is calledphagocytosis,which acts to defend the host against infection and injury.[3]
Macrophages are found in essentially all tissues,[4]where they patrol for potentialpathogensbyamoeboid movement.They take various forms (with various names) throughout the body (e.g.,histiocytes,Kupffer cells,alveolar macrophages,microglia,and others), but all are part of themononuclear phagocyte system.Besides phagocytosis, they play a critical role in nonspecific defense (innate immunity) and also help initiate specific defense mechanisms (adaptive immunity) by recruiting other immune cells such aslymphocytes.For example, they are important asantigen presenterstoT cells.In humans, dysfunctional macrophages cause severe diseases such aschronic granulomatous diseasethat result in frequent infections.
Beyond increasinginflammationand stimulating the immune system, macrophages also play an importantanti-inflammatoryrole and can decrease immune reactions through the release ofcytokines.Macrophages that encourage inflammation are called M1 macrophages, whereas those that decrease inflammation and encourage tissue repair are called M2 macrophages.[5]This difference is reflected in their metabolism; M1 macrophages have the unique ability to metabolizearginineto the "killer" moleculenitric oxide,whereas M2 macrophages have the unique ability to metabolize arginine to the "repair" moleculeornithine.[6]However, this dichotomy has been recently questioned as further complexity has been discovered.[7]
Human macrophages are about 21 micrometres (0.00083 in) in diameter[8]and are produced by the differentiation ofmonocytesin tissues. They can be identified usingflow cytometryorimmunohistochemical stainingby their specific expression of proteins such asCD14,CD40,CD11b,CD64,F4/80(mice)/EMR1(human),lysozymeM,MAC-1/MAC-3 andCD68.[9]
Macrophages were first discovered and named byÉlie Metchnikoff,a Russian Empire zoologist, in 1884.[10][11]
Structure[edit]
Types[edit]
A majority of macrophages are stationed at strategic points where microbial invasion or accumulation of foreign particles is likely to occur. These cells together as a group are known as themononuclear phagocyte systemand were previously known as the reticuloendothelial system. Each type of macrophage, determined by its location, has a specific name:
| Cell Name | Anatomical Location |
| Adipose tissue macrophages | Adipose tissue(fat) |
| Monocytes | Bone marrow/blood |
| Kupffer cells | Liver |
| Sinus histiocytes | Lymph nodes |
| Alveolar macrophages(dust cells) | Pulmonary alveoli |
| Tissue macrophages(histiocytes) leading togiant cells | Connective tissue |
| Microglia | Central nervous system |
| Hofbauer cells | Placenta |
| Intraglomerular mesangial cells[12] | Kidney |
| Osteoclasts[13] | Bone |
| Langerhans cells | Skin |
| Epithelioidcells | Granulomas |
| Red pulp macrophages(sinusoidallining cells) | Red pulp ofspleen |
| Peritoneal macrophages | Peritoneal cavity |
| perivascular Macrophages[14] | closely associated with blood vessels |
Investigations concerning Kupffer cells are hampered because in humans, Kupffer cells are only accessible for immunohistochemical analysis from biopsies or autopsies. From rats and mice, they are difficult to isolate, and after purification, only approximately 5 million cells can be obtained from one mouse.
Macrophages can expressparacrinefunctions within organs that are specific to the function of that organ. In thetestis,for example, macrophages have been shown to be able to interact withLeydig cellsby secreting25-hydroxycholesterol,anoxysterolthat can be converted totestosteroneby neighbouring Leydig cells.[15]Also, testicular macrophages may participate in creating an immune privileged environment in the testis, and in mediating infertility during inflammation of the testis.
Cardiac resident macrophages participate in electrical conduction viagap junctioncommunication with cardiacmyocytes.[16]
Macrophages can be classified on basis of the fundamental function and activation. According to this grouping, there areclassically activated (M1) macrophages,wound-healing macrophages (also known asalternatively-activated (M2) macrophages), andregulatory macrophages(Mregs).[17]
Development[edit]
Macrophages that reside in adult healthy tissues either derive from circulating monocytes or are established before birth and then maintained during adult life independently of monocytes.[18][19]By contrast, most of the macrophages that accumulate at diseased sites typically derive from circulating monocytes.[20]Leukocyte extravasationdescribesmonocyteentry into damaged tissue through theendotheliumofblood vesselsas they become macrophages. Monocytes are attracted to a damaged site by chemical substances throughchemotaxis,triggered by a range of stimuli including damaged cells, pathogens andcytokinesreleased by macrophages already at the site. At some sites such as the testis, macrophages have been shown to populate the organ through proliferation.[21]Unlike short-livedneutrophils,macrophages survive longer in the body, up to several months.
Function[edit]
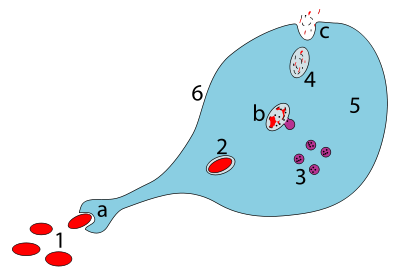
a.Ingestion through phagocytosis, a phagosome is formed
b.The fusion of lysosomes with the phagosome creates aphagolysosome;the pathogen is broken down by enzymes
c.Waste material is expelled orassimilated(the latter not pictured)
Parts:
1.Pathogens
2.Phagosome
3.Lysosomes
4.Waste material
5.Cytoplasm
6.Cell membrane
Phagocytosis[edit]
Macrophages areprofessional phagocytesand are highly specialized in removal of dying or dead cells and cellular debris. This role is important in chronic inflammation, as the early stages of inflammation are dominated by neutrophils, which are ingested by macrophages if they come of age (seeCD31for a description of this process).[22]
The neutrophils are at first attracted to a site, where they perform their function and die, before they or theirneutrophil extracellular trapsare phagocytized by the macrophages.[22][23]When at the site, the first wave of neutrophils, after the process of aging and after the first 48 hours, stimulate the appearance of the macrophages whereby these macrophages will then ingest the aged neutrophils.[22]
The removal of dying cells is, to a greater extent, handled byfixed macrophages,which will stay at strategic locations such as the lungs, liver,neural tissue,bone, spleen and connective tissue, ingesting foreign materials such as pathogens and recruiting additional macrophages if needed.[24]
When a macrophage ingests a pathogen, the pathogen becomes trapped in aphagosome,which then fuses with alysosome.Within thephagolysosome,enzymesand toxic peroxides digest the pathogen. However, some bacteria, such asMycobacterium tuberculosis,have become resistant to these methods of digestion.TyphoidalSalmonellaeinduce their own phagocytosis by host macrophages in vivo, and inhibit digestion by lysosomal action, thereby using macrophages for their own replication and causing macrophage apoptosis.[25]Macrophages can digest more than 100 bacteria before they finally die due to their own digestive compounds.
Role in innate immune response[edit]
When a pathogen invades, tissue resident macrophages are among the first cells to respond.[26]Two of the main roles of the tissue resident macrophages are to phagocytose incoming antigen and to secrete proinflammatory cytokines that induce inflammation and recruit other immune cells to the site.[27]
Phagocytosis of pathogens[edit]

Macrophages can internalize antigens through receptor-mediated phagocytosis.[28]Macrophages have a wide variety ofpattern recognition receptors(PRRs) that can recognizemicrobe-associated molecular patterns(MAMPs) from pathogens. Many PRRs, such astoll-like receptors(TLRs),scavenger receptors(SRs), C-type lectin receptors, among others, recognize pathogens for phagocytosis.[28]Macrophages can also recognize pathogens for phagocytosis indirectly throughopsonins,which are molecules that attach to pathogens and mark them for phagocytosis.[29]Opsonins can cause a stronger adhesion between the macrophage and pathogen during phagocytosis, hence opsonins tend to enhance macrophages’ phagocytic activity.[30]Bothcomplement proteinsand antibodies can bind to antigens and opsonize them. Macrophages have complement receptor 1 (CR1) and 3 (CR3) that recognize pathogen-bound complement proteins C3b and iC3b, respectively, as well as fragment crystallizable γ receptors (FcγRs) that recognize thefragment crystallizable (Fc) regionof antigen-boundimmunoglobulin G(IgG) antibodies.[29][31]When phagocytosing and digesting pathogens, macrophages go through arespiratory burstwhere more oxygen is consumed to supply the energy required for producing reactive oxygen species (ROS) and other antimicrobial molecules that digest the consumed pathogens.[27][32]
Cytokine secretion[edit]
Recognition of MAMPs by PRRs can activate tissue resident macrophages to secrete proinflammatory cytokines that recruit other immune cells. Among the PRRs, TLRs play a major role in signal transduction leading to cytokine production.[28]The binding of MAMPs to TLR triggers a series of downstream events that eventually activates transcription factorNF-κBand results in transcription of the genes for several proinflammatory cytokines, includingIL-1β,IL-6,TNF-α,IL-12B,andtype I interferonssuch as IFN-α and IFN-β.[33]Systemically, IL-1β, IL-6, and TNF-α induce fever and initiate the acute phase response in which the liver secretesacute phase proteins.[26][27][34]Locally, IL-1β and TNF-α cause vasodilation, where the gaps between blood vessel epithelial cells widen, and upregulation of cell surface adhesion molecules on epithelial cells to induceleukocyte extravasation.[26][27]
Neutrophilsare among the first immune cells recruited by macrophages to exit the blood via extravasation and arrive at the infection site.[34]Macrophages secrete manychemokinessuch asCXCL1,CXCL2,andCXCL8 (IL-8)that attract neutrophils to the site of infection.[26][34]After neutrophils have finished phagocytosing and clearing the antigen at the end of the immune response, they undergo apoptosis, and macrophages are recruited from blood monocytes to help clear apoptotic debris.[35]
Macrophages also recruit other immune cells such as monocytes, dendritic cells, natural killer cells, basophils, eosinophils, and T cells through chemokines such asCCL2,CCL4,CCL5,CXCL8,CXCL9,CXCL10,andCXCL11.[26][34]Along with dendritic cells, macrophages help activatenatural killer (NK) cellsthrough secretion oftype I interferons(IFN-α and IFN-β) andIL-12.IL-12 acts withIL-18to stimulate the production of proinflammatory cytokineinterferon gamma(IFN-γ) by NK cells, which serves as an important source of IFN-γ before the adaptive immune system is activated.[34][36]IFN-γ enhances the innate immune response by inducing a more aggressive phenotype in macrophages, allowing macrophages to more efficiently kill pathogens.[34]
Some of the T cell chemoattractants secreted by macrophages includeCCL5,CXCL9,CXCL10,andCXCL11.[26]
Role in adaptive immunity[edit]

Interactions with CD4+T Helper Cells[edit]
Macrophages are professional antigen presenting cells (APC), meaning they can present peptides from phagocytosed antigens on major histocompatibility complex (MHC) II molecules on their cell surface for T helper cells.[38]Macrophages are not primary activators of naïve T helper cells that have never been previously activated since tissue resident macrophages do not travel to the lymph nodes where naïve T helper cells reside.[39][40]Although macrophages are also found in secondary lymphoid organs like the lymph nodes, they do not reside in T cell zones and are not effective at activating naïve T helper cells.[39]The macrophages in lymphoid tissues are more involved in ingesting antigens and preventing them from entering the blood, as well as taking up debris from apoptotic lymphocytes.[39][41]Therefore, macrophages interact mostly with previously activated T helper cells that have left the lymph node and arrived at the site of infection or with tissue resident memory T cells.[40]
Macrophages supply both signals required for T helper cell activation: 1) Macrophages present antigen peptide-bound MHC class II molecule to be recognized by the correspondingT cell receptor(TCR), and 2) recognition of pathogens by PRRs induce macrophages to upregulate the co-stimulatory moleculesCD80andCD86(also known asB7) that binds toCD28on T helper cells to supply the co-stimulatory signal.[34][38]These interactions allow T helper cells to achieve full effector function and provide T helper cells with continued survival and differentiation signals preventing them from undergoing apoptosis due to lack of TCR signaling.[38]For example,IL-2signaling in T cells upregulates the expression of anti-apoptotic proteinBcl-2,but T cell production of IL-2 and the high-affinity IL-2 receptorIL-2RAboth require continued signal from TCR recognition of MHC-bound antigen.[34][42]
Activation[edit]
Macrophages can achieve different activation phenotypes through interactions with different subsets of T helper cells, such as TH1 and TH2.[17]Although there is a broad spectrum of macrophage activation phenotypes, there are two major phenotypes that are commonly acknowledged.[17]They are the classically activated macrophages, or M1 macrophages, and the alternatively activated macrophages, or M2 macrophages. M1 macrophages are proinflammatory, while M2 macrophages are mostly anti-inflammatory.[17]
Classical[edit]
TH1 cells play an important role in classical macrophage activation as part oftype 1 immune responseagainst intracellular pathogens (such asintracellular bacteria) that can survive and replicate inside host cells, especially those pathogens that replicate even after being phagocytosed by macrophages.[43]After the TCR of TH1 cells recognize specific antigen peptide-bound MHC class II molecules on macrophages, TH1 cells 1) secrete IFN-γ and 2) upregulate the expression ofCD40 ligand(CD40L), which binds toCD40on macrophages.[44][34]These 2 signals activate the macrophages and enhance their ability to kill intracellular pathogens through increased production of antimicrobial molecules such asnitric oxide(NO) andsuperoxide(O2-).[26][34]This enhancement of macrophages' antimicrobial ability by TH1 cells is known as classical macrophage activation, and the activated macrophages are known as classically activated macrophages, or M1 macrophages. The M1 macrophages in turn upregulates B7 molecules and antigen presentation through MHC class II molecules to provide signals that sustain T cell help.[44]The activation of TH1 and M1 macrophage is a positive feedback loop, with IFN-γ from TH1 cells upregulating CD40 expression on macrophages; the interaction between CD40 on the macrophages and CD40L on T cells activate macrophages to secrete IL-12; and IL-12 promotes more IFN-γ secretion from TH1 cells.[34][44]The initial contact between macrophage antigen-bound MHC II and TCR serves as the contact point between the two cells where most of the IFN-γ secretion and CD-40L on T cells concentrate to, so only macrophages directly interacting with TH1 cells are likely to be activated.[34]
In addition to activating M1 macrophages, TH1 cells expressFas ligand(FasL) andlymphotoxin beta(LT-β) to help kill chronically infected macrophages that can no longer kill pathogens.[34]The killing of chronically infected macrophages release pathogens to the extracellular space that can then be killed by other activated macrophages.[34]TH1 cells also help recruit more monocytes, the precursor to macrophages, to the infection site. TH1 secretionTNF-αandLT-αto make blood vessels easier for monocytes to bind to and exit.[34]TH1 secretion ofCCL2as a chemoattractant for monocytes.IL-3andGM-CSFreleased by TH1 cells stimulate more monocyte production in the bone marrow.[34]
When intracellular pathogens cannot be eliminated, such as in the case ofMycobacterium tuberculosis,the pathogen is contained through the formation ofgranuloma,an aggregation of infected macrophages surrounded by activated T cells.[45]The macrophages bordering the activated lymphocytes often fuse to form multinucleated giant cells that appear to have increased antimicrobial ability due to their proximity to TH1 cells, but over time, the cells in the center start to die and form necrotic tissue.[40][45]
Alternative[edit]
TH2 cells play an important role in alternative macrophage activation as part of type 2 immune response against large extracellular pathogens likehelminths.[34][46]TH2 cells secrete IL-4 and IL-13, which activate macrophages to become M2 macrophages, also known as alternatively activated macrophages.[46][47]M2 macrophages expressarginase-1,an enzyme that convertsargininetoornithineandurea.[46]Ornithine help increase smooth muscle contraction to expel the worm and also participates in tissue and wound repair. Ornithine can be further metabolized toproline,which is essential for synthesizingcollagen.[46]M2 macrophages can also decrease inflammation by producing IL-1 receptor antagonist (IL-1RA) and IL-1 receptors that do not lead to downstream inflammatory signaling (IL-1RII).[34][48]
Interactions with CD8+cytotoxic t cells[edit]
Another part of the adaptive immunity activation involves stimulating CD8+via cross presentation of antigens peptides on MHC class I molecules. Studies have shown that proinflammatory macrophages are capable of cross presentation of antigens on MHC class I molecules, but whether macrophage cross-presentation plays a role in naïve or memory CD8+T cell activation is still unclear.[27][49][41]
Interactions with B cells[edit]
Macrophages have been shown to secrete cytokines BAFF and APRIL, which are important for plasma cell isotype switching. APRIL and IL-6 secreted by macrophage precursors in the bone marrow help maintain survival of plasma cells homed to the bone marrow.[50]
Subtypes[edit]
There are several activated forms of macrophages.[17]In spite of a spectrum of ways to activate macrophages, there are two main groups designatedM1andM2.M1 macrophages: as mentioned earlier (previously referred to as classically activated macrophages),[52]M1 "killer" macrophages are activated byLPSandIFN-gamma,and secrete high levels ofIL-12and low levels ofIL-10.M1 macrophages have pro-inflammatory, bactericidal, and phagocytic functions.[53]In contrast, the M2 "repair" designation (also referred to as alternatively activated macrophages) broadly refers to macrophages that function in constructive processes like wound healing and tissue repair, and those that turn off damaging immune system activation by producing anti-inflammatory cytokines likeIL-10.M2 is the phenotype of resident tissue macrophages, and can be further elevated byIL-4.M2 macrophages produce high levels of IL-10,TGF-betaand low levels of IL-12. Tumor-associated macrophages are mainly of the M2 phenotype, and seem to actively promote tumor growth.[54]
Macrophages exist in a variety of phenotypes which are determined by the role they play in wound maturation. Phenotypes can be predominantly separated into two major categories; M1 and M2. M1 macrophages are the dominating phenotype observed in the early stages of inflammation and are activated by four key mediators: interferon-γ (IFN-γ), tumor necrosis factor (TNF), and damage associated molecular patterns (DAMPs). These mediator molecules create a pro-inflammatory response that in return produce pro-inflammatory cytokines like Interleukin-6 and TNF. Unlike M1 macrophages, M2 macrophages secrete an anti-inflammatory response via the addition of Interleukin-4 or Interleukin-13. They also play a role in wound healing and are needed for revascularization and reepithelialization. M2 macrophages are divided into four major types based on their roles: M2a, M2b, M2c, and M2d. How M2 phenotypes are determined is still up for discussion but studies have shown that their environment allows them to adjust to whichever phenotype is most appropriate to efficiently heal the wound.[53]
M2 macrophages are needed for vascular stability. They producevascular endothelial growth factor-AandTGF-β1.[53]There is a phenotype shift from M1 to M2 macrophages in acute wounds, however this shift is impaired for chronic wounds. This dysregulation results in insufficient M2 macrophages and its corresponding growth factors that aid in wound repair. With a lack of these growth factors/anti-inflammatory cytokines and an overabundance of pro-inflammatory cytokines from M1 macrophages chronic wounds are unable to heal in a timely manner. Normally, after neutrophils eat debris/pathogens they perform apoptosis and are removed. At this point, inflammation is not needed and M1 undergoes a switch to M2 (anti-inflammatory). However, dysregulation occurs as the M1 macrophages are unable/do not phagocytose neutrophils that have undergone apoptosis leading to increased macrophage migration and inflammation.[53]
Both M1 and M2 macrophages play a role in promotion ofatherosclerosis.M1 macrophages promote atherosclerosis by inflammation. M2 macrophages can remove cholesterol from blood vessels, but when the cholesterol is oxidized, the M2 macrophages becomeapoptoticfoam cellscontributing to theatheromatous plaqueof atherosclerosis.[55][56]
Role in muscle regeneration[edit]
The first step to understanding the importance of macrophages in muscle repair, growth, and regeneration is that there are two "waves" of macrophages with the onset of damageable muscle use– subpopulations that do and do not directly have an influence on repairing muscle. The initial wave is a phagocytic population that comes along during periods of increased muscle use that are sufficient to cause muscle membrane lysis and membrane inflammation, which can enter and degrade the contents of injured muscle fibers.[57][58][59]These early-invading, phagocytic macrophages reach their highest concentration about 24 hours following the onset of some form of muscle cell injury or reloading.[60]Their concentration rapidly declines after 48 hours.[58]The second group is the non-phagocytic types that are distributed near regenerative fibers. These peak between two and four days and remain elevated for several days during while muscle tissue is rebuilding.[58]The first subpopulation has no direct benefit to repairing muscle, while the second non-phagocytic group does.
It is thought that macrophages release soluble substances that influence the proliferation, differentiation, growth, repair, and regeneration of muscle, but at this time the factor that is produced to mediate these effects is unknown.[60]It is known that macrophages' involvement in promoting tissue repair is not muscle specific; they accumulate in numerous tissues during the healing process phase following injury.[61]
Role in wound healing[edit]
Macrophages are essential forwound healing.[62]They replacepolymorphonuclear neutrophilsas the predominant cells in the wound by day two after injury.[63]Attracted to the wound site by growth factors released by platelets and other cells,monocytesfrom the bloodstream enter the area through blood vessel walls.[64]Numbers of monocytes in the wound peak one to one and a half days after the injury occurs. Once they are in the wound site, monocytes mature into macrophages. Thespleencontains half the body's monocytes in reserve ready to be deployed to injured tissue.[65][66]
The macrophage's main role is to phagocytize bacteria and damaged tissue,[62]and they alsodebridedamaged tissue by releasing proteases.[67]Macrophages also secrete a number of factors such as growth factors and other cytokines, especially during the third and fourth post-wound days. These factors attract cells involved in the proliferation stage of healing to the area.[68]Macrophages may also restrain the contraction phase.[69]Macrophages are stimulated by the lowoxygencontent of their surroundings to produce factors that induce and speedangiogenesis[70]and they also stimulate cells that re-epithelialize the wound, create granulation tissue, and lay down a newextracellular matrix.[71][better source needed]By secreting these factors, macrophages contribute to pushing the wound healing process into the next phase.
Role in limb regeneration[edit]
Scientists have elucidated that as well as eating up material debris, macrophages are involved in the typicallimb regenerationin the salamander.[72][73]They found that removing the macrophages from asalamanderresulted in failure of limb regeneration and a scarring response.[72][73]
Role in iron homeostasis[edit]
As described above, macrophages play a key role in removing dying or dead cells and cellular debris.Erythrocyteshave a lifespan on average of 120 days and so are constantly being destroyed by macrophages in the spleen and liver. Macrophages will also engulfmacromolecules,and so play a key role in thepharmacokineticsofparenteral irons.[citation needed]
The iron that is released from the haemoglobin is either stored internally inferritinor is released into the circulation viaferroportin.In cases where systemic iron levels are raised, or where inflammation is present, raised levels ofhepcidinact on macrophage ferroportin channels, leading to iron remaining within the macrophages.[citation needed]
Role in pigment retainment[edit]

Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such astattoos), from extracellular space. In contrast to dendritic juncionalmelanocytes,whichsynthesize melanosomesand contain various stages of their development, the melanophages only accumulatephagocytosedmelanin in lysosome-like phagosomes.[74][75]This occurs repeatedly as the pigment from dead dermal macrophages is phagocytosed by their successors, preserving the tattoo in the same place.[76]
Role in tissue homeostasis[edit]
Every tissue harbors its own specialized population of resident macrophages, which entertain reciprocal interconnections with the stroma and functional tissue.[77][78]These resident macrophages are sessile (non-migratory), provide essential growth factors to support the physiological function of the tissue (e.g. macrophage-neuronal crosstalk in the guts),[79]and can actively protect the tissue from inflammatory damage.[80]
Nerve-associated macrophages[edit]
Nerve-associated macrophages or NAMs are those tissue-resident macrophages that are associated with nerves. Some of them are known to have an elongated morphology of up to 200μm[81]
Clinical significance[edit]
Due to their role in phagocytosis, macrophages are involved in many diseases of the immune system. For example, they participate in the formation ofgranulomas,inflammatory lesions that may be caused by a large number of diseases. Some disorders, mostly rare, of ineffective phagocytosis and macrophage function have been described, for example.[82]
As a host for intracellular pathogens[edit]
In their role as a phagocytic immune cell macrophages are responsible for engulfing pathogens to destroy them. Some pathogens subvert this process and instead live inside the macrophage. This provides an environment in which the pathogen is hidden from the immune system and allows it to replicate.[citation needed]
Diseases with this type of behaviour includetuberculosis(caused byMycobacterium tuberculosis) andleishmaniasis(caused byLeishmaniaspecies).[citation needed]
In order to minimize the possibility of becoming the host of an intracellular bacteria, macrophages have evolved defense mechanisms such as induction of nitric oxide and reactive oxygen intermediates,[83]which are toxic to microbes. Macrophages have also evolved the ability to restrict the microbe's nutrient supply and induceautophagy.[84]
Tuberculosis[edit]
Once engulfed by a macrophage, the causative agent of tuberculosis,Mycobacterium tuberculosis,[85]avoids cellular defenses and uses the cell to replicate. Recent evidence suggests that in response to the pulmonary infection ofMycobacterium tuberculosis,the peripheral macrophages matures into M1 phenotype. Macrophage M1 phenotype is characterized by increased secretion of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) and increased glycolytic activities essential for clearance of infection.[1]
Leishmaniasis[edit]
Upon phagocytosis by a macrophage, theLeishmaniaparasite finds itself in a phagocytic vacuole. Under normal circumstances, this phagocytic vacuole would develop into a lysosome and its contents would be digested.Leishmaniaalter this process and avoid being destroyed; instead, they make a home inside the vacuole.[citation needed]
Chikungunya[edit]
Infection of macrophages in joints is associated with local inflammation during and after the acute phase ofChikungunya(caused by CHIKV or Chikungunya virus).[86]
Others[edit]
Adenovirus(most common cause of pink eye) can remain latent in a host macrophage, with continued viral shedding 6–18 months after initial infection.[citation needed]
Brucella spp.can remain latent in a macrophage via inhibition ofphagosome–lysosomefusion; causesbrucellosis(undulant fever).[citation needed]
Legionella pneumophila,the causative agent ofLegionnaires' disease,also establishes residence within macrophages.[citation needed]
Heart disease[edit]
Macrophages are the predominant cells involved in creating the progressive plaque lesions ofatherosclerosis.[87]
Focal recruitment of macrophages occurs after the onset of acutemyocardial infarction.These macrophages function to remove debris, apoptotic cells and to prepare fortissue regeneration.[88]Macrophages protect against ischemia-induced ventricular tachycardia in hypokalemic mice.[89]
HIV infection[edit]
Macrophages also play a role inhuman immunodeficiency virus(HIV) infection. LikeT cells,macrophages can be infected with HIV, and even become a reservoir of ongoing virus replication throughout the body. HIV can enter the macrophage through binding of gp120 to CD4 and second membrane receptor, CCR5 (a chemokine receptor). Both circulating monocytes and macrophages serve as a reservoir for the virus.[90]Macrophages are better able to resist infection by HIV-1 than CD4+ T cells, although susceptibility to HIV infection differs among macrophage subtypes.[91]
Cancer[edit]
Macrophages can contribute to tumor growth and progression by promoting tumor cell proliferation and invasion, fostering tumor angiogenesis and suppressing antitumor immune cells.[92][93]Inflammatory compounds, such astumor necrosis factor(TNF)- Alpha released by the macrophages activate the gene switchnuclear factor-kappa B.NF-κB then enters the nucleus of a tumor cell and turns on production of proteins that stopapoptosisand promote cell proliferation and inflammation.[94]Moreover, macrophages serve as a source for many pro-angiogenic factors includingvascular endothelial factor(VEGF),tumor necrosis factor- Alpha(TNF- Alpha ),macrophage colony-stimulating factor(M-CSF/CSF1) andIL-1andIL-6,[95]contributing further to the tumor growth.
Macrophages have been shown to infiltrate a number of tumors. Their number correlates with poor prognosis in certain cancers, including cancers of breast, cervix, bladder, brain and prostate.[96][97]Some tumors can also produce factors, including M-CSF/CSF1,MCP-1/CCL2andAngiotensin II,that trigger the amplification and mobilization of macrophages in tumors.[98][99][100]Additionally, subcapsular sinus macrophages in tumor-draining lymph nodes can suppress cancer progression by containing the spread of tumor-derived materials.[101]
Cancer therapy[edit]
Experimental studies indicate that macrophages can affect all therapeutic modalities, includingsurgery,chemotherapy,radiotherapy,immunotherapyandtargeted therapy.[93][102][103]Macrophages can influence treatment outcomes both positively and negatively. Macrophages can be protective in different ways: they can remove dead tumor cells (in a process calledphagocytosis) following treatments that kill these cells; they can serve as drug depots for some anticancer drugs;[104]they can also be activated by some therapies to promote antitumor immunity.[105]Macrophages can also be deleterious in several ways: for example they can suppress various chemotherapies,[106][107]radiotherapies[108][109]and immunotherapies.[110][111]Because macrophages can regulate tumor progression, therapeutic strategies to reduce the number of these cells, or to manipulate their phenotypes, are currently being tested in cancer patients.[112][113]However, macrophages are also involved in antibody mediated cytotoxicity (ADCC) and this mechanism has been proposed to be important for certain cancer immunotherapy antibodies.[114]
Obesity[edit]
It has been observed that increased number of pro-inflammatory macrophages within obese adipose tissue contributes to obesity complications including insulin resistance and diabetes type 2.[115]
The modulation of the inflammatory state of adipose tissue macrophages has therefore been considered a possible therapeutic target to treat obesity-related diseases.[116]Although adipose tissue macrophages are subject to anti-inflammatory homeostatic control by sympathetic innervation, experiments usingADRB2 geneknockout mice indicate that this effect is indirectly exerted through the modulation of adipocyte function, and not through directBeta-2 adrenergic receptoractivation, suggesting that adrenergic stimulation of macrophages may be insufficient to impact adipose tissue inflammation or function in obesity.[117]
Within the fat (adipose) tissue ofCCR2deficientmice,there is an increased number ofeosinophils,greater alternative macrophage activation, and a propensity towards type 2cytokineexpression. Furthermore, this effect was exaggerated when the mice becameobesefrom a high fat diet.[118]This is partially caused by a phenotype switch of macrophages induced bynecrosisof fat cells (adipocytes). In an obese individual some adipocytes burst and undergo necrotic death, which causes the residential M2 macrophages to switch to M1 phenotype. This is one of the causes of a low-grade systemic chronic inflammatory state associated with obesity.[119][120]
Intestinal macrophages[edit]
Though very similar in structure to tissue macrophages, intestinal macrophages have evolved specific characteristics and functions given their natural environment, which is in the digestive tract. Macrophages and intestinal macrophages have high plasticity causing their phenotype to be altered by their environments.[121]Like macrophages, intestinal macrophages are differentiated monocytes, though intestinal macrophages have to coexist with themicrobiomein the intestines. This is a challenge considering the bacteria found in the gut are not recognized as "self" and could be potential targets for phagocytosis by the macrophage.[122]
To prevent the destruction of the gut bacteria, intestinal macrophages have developed key differences compared to other macrophages. Primarily, intestinal macrophages do not induce inflammatory responses. Whereas tissue macrophages release various inflammatory cytokines, such as IL-1, IL-6 and TNF-α, intestinal macrophages do not produce or secrete inflammatory cytokines. This change is directly caused by the intestinal macrophages environment. Surrounding intestinal epithelial cells releaseTGF-β,which induces the change from proinflammatory macrophage to noninflammatory macrophage.[122]
Even though the inflammatory response is downregulated in intestinal macrophages, phagocytosis is still carried out. There is no drop off in phagocytosis efficiency as intestinal macrophages are able to effectively phagocytize the bacteria,S. typhimuriumandE. coli,but intestinal macrophages still do not release cytokines, even after phagocytosis. Also, intestinal macrophages do not express lipopolysaccharide (LPS), IgA, or IgG receptors.[123]The lack of LPS receptors is important for the gut as the intestinal macrophages do not detect the microbe-associated molecular patterns(MAMPS/PAMPS)of the intestinal microbiome. Nor do they express IL-2 and IL-3 growth factor receptors.[122]
Role in disease[edit]
Intestinal macrophages have been shown to play a role ininflammatory bowel disease(IBD), such asCrohn's disease(CD) andulcerative colitis(UC). In a healthy gut, intestinal macrophages limit the inflammatory response in the gut, but in a disease-state, intestinal macrophage numbers and diversity are altered. This leads to inflammation of the gut and disease symptoms of IBD. Intestinal macrophages are critical in maintaining guthomeostasis.The presence of inflammation or pathogen alters this homeostasis, and concurrently alters the intestinal macrophages.[124]There has yet to be a determined mechanism for the alteration of the intestinal macrophages by recruitment of new monocytes or changes in the already present intestinal macrophages.[123]
Additionally, a new study reveals macrophages limit iron access to bacteria by releasing extracellular vesicles, improving sepsis outcomes.[125]
Media[edit]
-
An active J774 macrophage is seen taking up fourconidiain a co-operative manner. The J774 cells were treated with 5ng/mlinterferon-γone night before filming with conidia. Observations were made every 30s over a 2.5hr period.
-
Two highly activealveolar macrophagescan be seen ingestingconidia.Time lapse is 30s per frame over 2.5hr.
History[edit]
This sectionneeds expansion.You can help byadding to it.(March 2018) |
Macrophages were first discovered late in the 19th century byÉlie Metchnikoff.[126]Élie Metchnikoff revolutionized the branch of macrophages by combining philosophical insights and the evolutionary study of life.[127]Later on, Van Furth during the 1960s proposed the idea that circulating blood monocytes in adults allowed for the origin of all tissue macrophages.[128]In recent years, publishing regarding macrophages has led people to believe that multiple resident tissue macrophages are independent of the blood monocytes as it is formed during the embryonic stage of development.[129]Within the 21st century, all the ideas concerning the origin of macrophages (present in tissues) are compiled together to suggest that physiologically complex organisms, form macrophages independently by mechanisms that don't have to depend on the blood monocytes.[130]
See also[edit]
References[edit]
- ^abMahla RS, Kumar A, Tutill HJ, Krishnaji ST, Sathyamoorthy B, Noursadeghi M, et al. (January 2021). "NIX-mediated mitophagy regulate metabolic reprogramming in phagocytic cells during mycobacterial infection".Tuberculosis.126(January): 102046.doi:10.1016/j.tube.2020.102046.PMID33421909.S2CID231437641.
- ^"Regenerative Medicine Partnership in Education".Archived fromthe originalon 25 April 2015.Retrieved7 May2015.
- ^Nahrendorf M, Hoyer FF, Meerwaldt AE, van Leent MM, Senders ML, Calcagno C, et al. (October 2020)."Imaging Cardiovascular and Lung Macrophages With the Positron Emission Tomography Sensor64Cu-Macrin in Mice, Rabbits, and Pigs ".Circulation: Cardiovascular Imaging.13(10): e010586.doi:10.1161/CIRCIMAGING.120.010586.PMC7583675.PMID33076700.
- ^Ovchinnikov DA (September 2008)."Macrophages in the embryo and beyond: much more than just giant phagocytes".Genesis.46(9): 447–462.doi:10.1002/dvg.20417.PMID18781633.S2CID38894501.
Macrophages are present essentially in all tissues, beginning with embryonic development and, in addition to their role in host defense and in the clearance of apoptotic cells, are being increasingly recognized for their trophic function and role in regeneration.
- ^Mills CD (2012). "M1 and M2 Macrophages: Oracles of Health and Disease".Critical Reviews in Immunology.32(6): 463–488.doi:10.1615/CritRevImmunol.v32.i6.10.PMID23428224.
- ^Murphy K, Weaver C (2006).Janeway's immunobiology.Garland Science, New York. pp. 464, 904.ISBN978-0-8153-4551-0.
- ^Ransohoff RM (July 2016). "A polarizing question: do M1 and M2 microglia exist?".Nature Neuroscience.19(8): 987–991.doi:10.1038/nn.4338.PMID27459405.S2CID27541569.
- ^Krombach F, Münzing S, Allmeling AM, Gerlach JT, Behr J, Dörger M (September 1997)."Cell size of alveolar macrophages: an interspecies comparison".Environmental Health Perspectives.105(Suppl 5): 1261–1263.doi:10.2307/3433544.JSTOR3433544.PMC1470168.PMID9400735.
- ^Khazen W, M'bika JP, Tomkiewicz C, Benelli C, Chany C, Achour A, et al. (October 2005)."Expression of macrophage-selective markers in human and rodent adipocytes".FEBS Letters.579(25): 5631–5634.doi:10.1016/j.febslet.2005.09.032.PMID16213494.S2CID6066984.
- ^Zalkind S (2001).Ilya Mechnikov: His Life and Work.Honolulu, Hawaii: University Press of the Pacific. pp. 78, 210.ISBN978-0-89875-622-7.
- ^Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. (September 2018)."Macrophage plasticity, polarization, and function in health and disease".Journal of Cellular Physiology.233(9): 6425–6440.doi:10.1002/jcp.26429.PMID29319160.S2CID3621509.
- ^Lote CJ.Principles of Renal Physiology, 5th edition.Springer. p. 37.
- ^Shirazi S, Ravindran S, Cooper LF (December 2022)."Topography-mediated immunomodulation in osseointegration; Ally or Enemy".Biomaterials.291:121903.doi:10.1016/j.biomaterials.2022.121903.PMC10148651.PMID36410109.
- ^Siret C, van Lessen M, Bavais J, Jeong HW, Reddy Samawar SK, Kapupara K, et al. (2022)."Deciphering the heterogeneity of the Lyve1+ perivascular macrophages in the mouse brain".Nature Communications.13(1): 7366.Bibcode:2022NatCo..13.7366S.doi:10.1038/s41467-022-35166-9.PMC9712536.PMID36450771.
- ^Nes WD, Lukyanenko YO, Jia ZH, Quideau S, Howald WN, Pratum TK, et al. (March 2000)."Identification of the lipophilic factor produced by macrophages that stimulates steroidogenesis".Endocrinology.141(3): 953–958.doi:10.1210/endo.141.3.7350.PMID10698170.
- ^Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, et al. (April 2017)."Macrophages Facilitate Electrical Conduction in the Heart".Cell.169(3): 510–522.e20.doi:10.1016/j.cell.2017.03.050.PMC5474950.PMID28431249.
- ^abcdeMosser DM, Edwards JP (December 2008)."Exploring the full spectrum of macrophage activation".Nature Reviews. Immunology.8(12): 958–969.doi:10.1038/nri2448.PMC2724991.PMID19029990.
- ^Perdiguero EG, Geissmann F (January 2016)."The development and maintenance of resident macrophages".Nature Immunology.17(1): 2–8.doi:10.1038/ni.3341.PMC4950995.PMID26681456.
- ^Ginhoux F, Guilliams M (March 2016)."Tissue-Resident Macrophage Ontogeny and Homeostasis".Immunity.44(3): 439–449.doi:10.1016/j.immuni.2016.02.024.PMID26982352.
- ^Pittet MJ, Nahrendorf M, Swirski FK (June 2014)."The journey from stem cell to macrophage".Annals of the New York Academy of Sciences.1319(1): 1–18.Bibcode:2014NYASA1319....1P.doi:10.1111/nyas.12393.PMC4074243.PMID24673186.
- ^Wang M, Yang Y, Cansever D, Wang Y, Kantores C, Messiaen S, et al. (January 2021)."Two populations of self-maintaining monocyte-independent macrophages exist in adult epididymis and testis".Proceedings of the National Academy of Sciences of the United States of America.118(1): e2013686117.Bibcode:2021PNAS..11813686W.doi:10.1073/pnas.2013686117.PMC7817195.PMID33372158.
- ^abcEming SA, Krieg T, Davidson JM (March 2007)."Inflammation in wound repair: molecular and cellular mechanisms".The Journal of Investigative Dermatology.127(3): 514–25.doi:10.1038/sj.jid.5700701.PMID17299434.
=Monocytes/macrophages. Unless stimuli for neutrophil recruitment persist at the wound site, the neutrophil infiltration ceases after few days, and expended neutrophils are themselves phagocytosed by macrophages, which are present at the wound side within 2 days after injury.
- ^Monteith AJ, Miller JM, Maxwell CN, Chazin WJ, Skaar EP (September 2021)."Neutrophil extracellular traps enhance macrophage killing of bacterial pathogens".Science Advances.7(37): eabj2101.Bibcode:2021SciA....7.2101M.doi:10.1126/sciadv.abj2101.PMC8442908.PMID34516771.
- ^Verma N, Saraf S (15 November 2017)."A Role of Macrophages: An Overview".Journal of Drug Delivery and Therapeutics.7(6): 91–103.doi:10.22270/jddt.v7i6.1521.ISSN2250-1177.
- ^YashRoy RC (2000)."Hijacking of Macrophages by Salmonella (310r) Through 'Types III' Secretion Like Exocytotic Signalling: A Mechanism for Infection of Chicken Ileum".Indian Journal of Poultry Science.35(3): 276–281.
- ^abcdefgArango Duque G, Descoteaux A (7 October 2014)."Macrophage cytokines: involvement in immunity and infectious diseases".Frontiers in Immunology.5:491.doi:10.3389/fimmu.2014.00491.PMC4188125.PMID25339958.
- ^abcdePunt J, Stranford S, Jones P, Owen J (25 May 2018).Kuby Immunology(8th ed.). New York, New York: W. H. Freeman.ISBN978-1-4641-8978-4.
- ^abcFu YL, Harrison RE (29 April 2021)."Microbial Phagocytic Receptors and Their Potential Involvement in Cytokine Induction in Macrophages".Frontiers in Immunology.12:662063.doi:10.3389/fimmu.2021.662063.PMC8117099.PMID33995386.
- ^abHirayama D, Iida T, Nakase H (December 2017)."The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis".International Journal of Molecular Sciences.19(1): 92.doi:10.3390/ijms19010092.PMC5796042.PMID29286292.
- ^Uribe-Querol E, Rosales C (2 June 2020)."Phagocytosis: Our Current Understanding of a Universal Biological Process".Frontiers in Immunology.11:1066.doi:10.3389/fimmu.2020.01066.PMC7280488.PMID32582172.
- ^Law SK (1 January 1988). "C3 receptors on macrophages".Journal of Cell Science. Supplement.9(Supplement_9): 67–97.doi:10.1242/jcs.1988.Supplement_9.4.PMID2978518.S2CID29387085.
- ^Forman HJ, Torres M (December 2002). "Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling".American Journal of Respiratory and Critical Care Medicine.166(12 Pt 2): S4–S8.doi:10.1164/rccm.2206007.PMID12471082.S2CID22246117.
- ^Liu T, Zhang L, Joo D, Sun SC (14 July 2017)."NF-κB signaling in inflammation".Signal Transduction and Targeted Therapy.2(1): 17023–.doi:10.1038/sigtrans.2017.23.PMC5661633.PMID29158945.
- ^abcdefghijklmnopqrMurphy K, Weaver C, Berg L (2022).Janeway's Immunobiology(10th ed.). New York, New York: W. W. Norton & Company.ISBN978-0-393-88487-6.
- ^Eming SA, Krieg T, Davidson JM (March 2007)."Inflammation in wound repair: molecular and cellular mechanisms".The Journal of Investigative Dermatology.127(3): 514–525.doi:10.1038/sj.jid.5700701.PMID17299434.
- ^Mezouar S, Mege JL (July 2020). "Changing the paradigm of IFN-γ at the interface between innate and adaptive immunity: Macrophage-derived IFN-γ".Journal of Leukocyte Biology.108(1): 419–426.doi:10.1002/JLB.4MIR0420-619RR.PMID32531848.S2CID219622032.
- ^Kress H, Stelzer EH, Holzer D, Buss F, Griffiths G, Rohrbach A (July 2007)."Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity".Proceedings of the National Academy of Sciences of the United States of America.104(28): 11633–11638.Bibcode:2007PNAS..10411633K.doi:10.1073/pnas.0702449104.PMC1913848.PMID17620618.
- ^abcGuerriero JL (2019). "Macrophages: Their Untold Story in T Cell Activation and Function".International Review of Cell and Molecular Biology.342.Elsevier: 73–93.doi:10.1016/bs.ircmb.2018.07.001.ISBN978-0-12-815381-9.PMID30635094.
- ^abcItano AA, Jenkins MK (August 2003). "Antigen presentation to naive CD4 T cells in the lymph node".Nature Immunology.4(8): 733–739.doi:10.1038/ni957.PMID12888794.S2CID10305140.
- ^abcMurphy K, Weaver C (2016).Janeway's Immunobiology(9th ed.). New York, New York: Garland Science. pp. 363–364.ISBN978-0-8153-4505-3.
- ^abGray EE, Cyster JG (2012)."Lymph node macrophages".Journal of Innate Immunity.4(5–6): 424–436.doi:10.1159/000337007.PMC3574571.PMID22488251.
- ^Abbas AK (September 2020)."The Surprising Story of IL-2".The American Journal of Pathology.190(9): 1776–1781.doi:10.1016/j.ajpath.2020.05.007.PMID32828360.S2CID221280663.
- ^Annunziato F, Romagnani C, Romagnani S (March 2015)."The 3 major types of innate and adaptive cell-mediated effector immunity".The Journal of Allergy and Clinical Immunology.135(3): 626–635.doi:10.1016/j.jaci.2014.11.001.PMID25528359.
- ^abcCai H, Zhang Y, Wang J, Gu J (23 June 2021)."Defects in Macrophage Reprogramming in Cancer Therapy: The Negative Impact of PD-L1/PD-1".Frontiers in Immunology.12:690869.doi:10.3389/fimmu.2021.690869.PMC8260839.PMID34248982.
- ^abHilhorst M, Shirai T, Berry G, Goronzy JJ, Weyand CM (2014)."T cell-macrophage interactions and granuloma formation in vasculitis".Frontiers in Immunology.5:432.doi:10.3389/fimmu.2014.00432.PMC4162471.PMID25309534.
- ^abcdRolot M, Dewals BG (2 July 2018)."Macrophage Activation and Functions during Helminth Infection: Recent Advances from the Laboratory Mouse".Journal of Immunology Research.2018:2790627.doi:10.1155/2018/2790627.PMC6051086.PMID30057915.
- ^Gordon S (January 2003). "Alternative activation of macrophages".Nature Reviews. Immunology.3(1): 23–35.doi:10.1038/nri978.PMID12511873.S2CID23185583.
- ^Peters VA, Joesting JJ, Freund GG (August 2013)."IL-1 receptor 2 (IL-1R2) and its role in immune regulation".Brain, Behavior, and Immunity.32:1–8.doi:10.1016/j.bbi.2012.11.006.PMC3610842.PMID23195532.
- ^Muntjewerff EM, Meesters LD, van den Bogaart G (8 July 2020)."Antigen Cross-Presentation by Macrophages".Frontiers in Immunology.11:1276.doi:10.3389/fimmu.2020.01276.PMC7360722.PMID32733446.
- ^Xu W, Banchereau J (January 2014)."The antigen presenting cells instruct plasma cell differentiation".Frontiers in Immunology.4:504.doi:10.3389/fimmu.2013.00504.PMC3880943.PMID24432021.
- ^Cotran RS, Kumar V, Collins T (1999).Robbins Pathologic Basis of Disease.Philadelphia: W.B Saunders Company.ISBN978-0-7216-7335-6.
- ^"The lymphocyte story".New Scientist(1605).Retrieved13 September2007.
- ^abcdHesketh M, Sahin KB, West ZE, Murray RZ (July 2017)."Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing".International Journal of Molecular Sciences.18(7): 1545.doi:10.3390/ijms18071545.PMC5536033.PMID28714933.
- ^Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A (July 2013). "Tumor associated macrophages and neutrophils in tumor progression".Journal of Cellular Physiology.228(7): 1404–1412.doi:10.1002/jcp.24260.PMID23065796.S2CID41189572.
- ^Hotamisligil GS (April 2010)."Endoplasmic reticulum stress and atherosclerosis".Nature Medicine.16(4): 396–399.doi:10.1038/nm0410-396.PMC2897068.PMID20376052.
- ^Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, et al. (April 2012)."Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation".The Journal of Biological Chemistry.287(15): 11629–11641.doi:10.1074/jbc.M111.338673.PMC3320912.PMID22356914.
- ^Krippendorf BB, Riley DA (January 1993). "Distinguishing unloading- versus reloading-induced changes in rat soleus muscle".Muscle & Nerve.16(1): 99–108.doi:10.1002/mus.880160116.PMID8423838.S2CID23012375.
- ^abcSt Pierre BA, Tidball JG (July 1994). "Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension".Journal of Applied Physiology.77(1): 290–297.doi:10.1152/jappl.1994.77.1.290.PMID7961247.
- ^Tidball JG, Berchenko E, Frenette J (April 1999)."Macrophage invasion does not contribute to muscle membrane injury during inflammation".Journal of Leukocyte Biology.65(4): 492–498.doi:10.1002/jlb.65.4.492.PMID10204578.S2CID23315528.
- ^abSchiaffino S, Partridge T (2008).Skeletal Muscle Repair and Regeneration.Advances in Muscle Research. Vol. 3.
- ^Bréchot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, et al. (2008)."Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice".PLOS ONE.3(12): e3950.Bibcode:2008PLoSO...3.3950B.doi:10.1371/journal.pone.0003950.PMC2597179.PMID19079608.
- ^abde la Torre J., Sholar A. (2006).Wound healing: Chronic wounds.Emedicine. Accessed 20 January 2008.
- ^"The phases of cutaneous wound healing"(PDF).Expert Reviews in Molecular Medicine.5.Cambridge University Press. 21 March 2003. Archived fromthe original(PDF)on 17 December 2008.
- ^Lorenz HP, Longaker MT (2003)."Wounds: biology, pathology, and management."(PDF).In Li M, Norton JA, Bollinger RR, Chang AE, Lowry SF, Mulvihill SJ, Pass HI, Thompson RW (eds.).Essential practice of surgery.New York, NY: Springer. pp. 77–88.ISBN978-0-387-22744-3.Archived fromthe original(PDF)on 31 October 2005.
- ^Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. (July 2009)."Identification of splenic reservoir monocytes and their deployment to inflammatory sites".Science.325(5940): 612–616.Bibcode:2009Sci...325..612S.doi:10.1126/science.1175202.PMC2803111.PMID19644120.
- ^Jia T, Pamer EG (July 2009)."Immunology. Dispensable but not irrelevant".Science.325(5940): 549–550.Bibcode:2009Sci...325..549J.doi:10.1126/science.1178329.PMC2917045.PMID19644100.
- ^Deodhar AK, Rana RE (1997)."Surgical physiology of wound healing: a review".Journal of Postgraduate Medicine.43(2): 52–56.PMID10740722.
- ^Rosenberg L., de la Torre J. (2006).Wound Healing, Growth Factors.Emedicine. Accessed 20 January 2008.
- ^Newton PM, Watson JA, Wolowacz RG, Wood EJ (August 2004). "Macrophages restrain contraction of an in vitro wound healing model".Inflammation.28(4): 207–214.doi:10.1023/B:IFLA.0000049045.41784.59.PMID15673162.S2CID9612298.
- ^Greenhalgh DG (September 1998). "The role of apoptosis in wound healing".The International Journal of Biochemistry & Cell Biology.30(9): 1019–1030.doi:10.1016/S1357-2725(98)00058-2.PMID9785465.
- ^Stashak TS, Farstvedt E, Othic A (June 2004). "Update on wound dressings: Indications and best use".Clinical Techniques in Equine Practice.3(2): 148–163.doi:10.1053/j.ctep.2004.08.006.
- ^abSouppouris A (23 May 2013)."Scientists identify cell that could hold the secret to limb regeneration".the verge.
Researchers have identified a cell that aids limb regrowth in Salamanders. Macrophages are a type of repairing cell that devour dead cells and pathogens, and trigger other immune cells to respond to pathogens.
- ^abGodwin JW, Pinto AR, Rosenthal NA (June 2013)."Macrophages are required for adult salamander limb regeneration".Proceedings of the National Academy of Sciences of the United States of America.110(23): 9415–9420.Bibcode:2013PNAS..110.9415G.doi:10.1073/pnas.1300290110.PMC3677454.PMID23690624.
- ^Mishima Y (October 1967)."Lysosomes in malanin phagocytosis and synthesis".Nature.216(5110): 67.Bibcode:1967Natur.216...67M.doi:10.1038/216067a0.PMID6050674.S2CID4285140.
- ^Mishima Y (January 1966)."Cellular and subcellular differentiation of melanin phagocytosis and synthesis by lysosomal and melanosomal activity".The Journal of Investigative Dermatology.46(1): 70–75.doi:10.1038/jid.1966.11.PMID5905254.
- ^Baranska A, Shawket A, Jouve M, Baratin M, Malosse C, Voluzan O, et al. (April 2018)."Unveiling skin macrophage dynamics explains both tattoo persistence and strenuous removal".The Journal of Experimental Medicine.215(4): 1115–1133.doi:10.1084/jem.20171608.PMC5881467.PMID29511065.
- ^Okabe Y, Medzhitov R (May 2014)."Tissue-specific signals control reversible program of localization and functional polarization of macrophages".Cell.157(4): 832–844.doi:10.1016/j.cell.2014.04.016.PMC4137874.PMID24792964.
- ^Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, et al. (December 2014)."Environment drives selection and function of enhancers controlling tissue-specific macrophage identities".Cell.159(6): 1327–1340.doi:10.1016/j.cell.2014.11.023.PMC4364385.PMID25480297.
- ^Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, et al. (July 2014)."Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility".Cell.158(2): 300–313.doi:10.1016/j.cell.2014.04.050.PMC4149228.PMID25036630.
- ^Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN (April 2019)."Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage".Cell.177(3): 541–555.e17.doi:10.1016/j.cell.2019.02.028.PMC6474841.PMID30955887.
- ^Kolter J, Kierdorf K, Henneke P (January 2020)."Origin and Differentiation of Nerve-Associated Macrophages".Journal of Immunology.204(2): 271–279.doi:10.4049/jimmunol.1901077.PMID31907269.S2CID210043405.
- ^Wolf AJ, Underhill DM (2014). "Phagocytosis".Macrophages: Biology and Role in the Pathology of Diseases.Springer New York. pp. 91–109.doi:10.1007/978-1-4939-1311-4_5.ISBN978-1-4939-1310-7.
- ^Herb M, Schramm M (February 2021)."Functions of ROS in Macrophages and Antimicrobial Immunity".Antioxidants.10(2): 313.doi:10.3390/antiox10020313.PMC7923022.PMID33669824.
- ^Weiss G, Schaible UE (March 2015)."Macrophage defense mechanisms against intracellular bacteria".Immunological Reviews.264(1): 182–203.doi:10.1111/imr.12266.PMC4368383.PMID25703560.
- ^Ryan KJ, Ray CG, eds. (2004).Sherris Medical Microbiology(4th ed.). McGraw Hill.ISBN978-0-8385-8529-0.
- ^Dupuis-Maguiraga L, Noret M, Brun S, Le Grand R, Gras G, Roques P (2012)."Chikungunya disease: infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia".PLOS Neglected Tropical Diseases.6(3): e1446.doi:10.1371/journal.pntd.0001446.PMC3313943.PMID22479654.
- ^Lucas AD, Greaves DR (November 2001). "Atherosclerosis: role of chemokines and macrophages".Expert Reviews in Molecular Medicine.3(25): 1–18.doi:10.1017/S1462399401003696.PMID14585150.S2CID8952545.
- ^Frantz S, Nahrendorf M (May 2014)."Cardiac macrophages and their role in ischaemic heart disease".Cardiovascular Research.102(2): 240–248.doi:10.1093/cvr/cvu025.PMC3989449.PMID24501331.
- ^Grune J, Lewis AJ, Yamazoe M, Hulsmans M, Rohde D, Xiao L, et al. (July 2022)."Neutrophils incite and macrophages avert electrical storm after myocardial infarction".Nature Cardiovascular Research.1(7): 649–664.doi:10.1038/s44161-022-00094-w.PMC9410341.PMID36034743.S2CID250475623.
- ^Bol SM, Cobos-Jiménez V, Kootstra NA, van't Wout AB (February 2011). "Macrophage".Future Virology.6(2): 187–208.doi:10.2217/fvl.10.93.
- ^Koppensteiner H, Brack-Werner R, Schindler M (October 2012)."Macrophages and their relevance in Human Immunodeficiency Virus Type I infection".Retrovirology.9(1): 82.doi:10.1186/1742-4690-9-82.PMC3484033.PMID23035819.
- ^Qian BZ, Pollard JW (April 2010)."Macrophage diversity enhances tumor progression and metastasis".Cell.141(1): 39–51.doi:10.1016/j.cell.2010.03.014.PMC4994190.PMID20371344.
- ^abEngblom C, Pfirschke C, Pittet MJ (July 2016). "The role of myeloid cells in cancer therapies".Nature Reviews. Cancer.16(7): 447–462.doi:10.1038/nrc.2016.54.PMID27339708.S2CID21924175.
- ^Stix G (July 2007). "A malignant flame. Understanding chronic inflammation, which contributes to heart disease, Alzheimer's and a variety of other ailments, may be a key to unlocking the mysteries of cancer".Scientific American.297(1): 60–67.Bibcode:2007SciAm.297a..60S.doi:10.1038/scientificamerican0707-60.PMID17695843.
- ^Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. (December 2006). "Macrophages regulate the angiogenic switch in a mouse model of breast cancer".Cancer Research.66(23): 11238–11246.doi:10.1158/0008-5472.can-06-1278.PMID17114237.S2CID12722658.
- ^Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002; 196:254–65.
- ^de Groot AE (July 2018)."In vitro human tumor-associated macrophage model implicates macrophage proliferation as a mechanism for maintaining tumor-associated macrophage populations".Cancer Research.78(13 Supplement): 4060.doi:10.1158/1538-7445.AM2018-4060.S2CID80769044.
- ^Lin EY, Nguyen AV, Russell RG, Pollard JW (March 2001)."Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy".The Journal of Experimental Medicine.193(6): 727–740.doi:10.1084/jem.193.6.727.PMC2193412.PMID11257139.
- ^Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. (June 2011)."CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis".Nature.475(7355): 222–225.doi:10.1038/nature10138.PMC3208506.PMID21654748.
- ^Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, et al. (February 2013)."Angiotensin II drives the production of tumor-promoting macrophages".Immunity.38(2): 296–308.doi:10.1016/j.immuni.2012.10.015.PMC3582771.PMID23333075.
- ^Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, et al. (April 2016)."SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions".Science.352(6282): 242–246.Bibcode:2016Sci...352..242P.doi:10.1126/science.aaf1328.PMC4960636.PMID26989197.
- ^Mantovani A, Allavena P (April 2015)."The interaction of anticancer therapies with tumor-associated macrophages".The Journal of Experimental Medicine.212(4): 435–445.doi:10.1084/jem.20150295.PMC4387285.PMID25753580.
- ^De Palma M, Lewis CE (March 2013)."Macrophage regulation of tumor responses to anticancer therapies".Cancer Cell.23(3): 277–286.doi:10.1016/j.ccr.2013.02.013.PMID23518347.
- ^Miller MA, Zheng YR, Gadde S, Pfirschke C, Zope H, Engblom C, et al. (October 2015)."Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug".Nature Communications.6:8692.Bibcode:2015NatCo...6.8692M.doi:10.1038/ncomms9692.PMC4711745.PMID26503691.
- ^Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. (November 2013)."Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy".Cancer Cell.24(5): 589–602.doi:10.1016/j.ccr.2013.09.014.PMID24209604.
- ^Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. (November 2014)."Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells".Cancer Cell.26(5): 623–637.doi:10.1016/j.ccell.2014.09.006.PMC4254570.PMID25446896.
- ^DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. (June 2011)."Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy".Cancer Discovery.1(1): 54–67.doi:10.1158/2159-8274.CD-10-0028.PMC3203524.PMID22039576.
- ^Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM (May 2015)."TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy".Cancer Immunology Research.3(5): 518–525.doi:10.1158/2326-6066.CIR-14-0232.PMC4420686.PMID25716473.
- ^Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG (July 2010)."Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation".Cancer Research.70(14): 5679–5685.doi:10.1158/0008-5472.CAN-09-4446.PMC2918387.PMID20631066.
- ^Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, et al. (May 2017)."In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy".Science Translational Medicine.9(389): eaal3604.doi:10.1126/scitranslmed.aal3604.PMC5734617.PMID28490665.
- ^Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. (September 2014)."CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models".Cancer Research.74(18): 5057–5069.doi:10.1158/0008-5472.CAN-13-3723.PMC4182950.PMID25082815.
- ^Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. (June 2014)."Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy".Cancer Cell.25(6): 846–859.doi:10.1016/j.ccr.2014.05.016.PMID24898549.
- ^Ruffell B, Coussens LM (April 2015)."Macrophages and therapeutic resistance in cancer".Cancer Cell.27(4): 462–472.doi:10.1016/j.ccell.2015.02.015.PMC4400235.PMID25858805.
- ^Sharma N, Vacher J, Allison JP (May 2019)."TLR1/2 ligand enhances antitumor efficacy of CTLA-4 blockade by increasing intratumoral Treg depletion".Proceedings of the National Academy of Sciences of the United States of America.116(21): 10453–10462.Bibcode:2019PNAS..11610453S.doi:10.1073/pnas.1819004116.PMC6534983.PMID31076558.
- ^Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue "Journal of Clinical Investigation2003; 112:1796–808.
- ^Guilherme A, Henriques F, Bedard AH, Czech MP (April 2019)."Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus".Nature Reviews. Endocrinology.15(4): 207–225.doi:10.1038/s41574-019-0165-y.PMC7073451.PMID30733616.
- ^Petkevicius K, Bidault G, Virtue S, Newland SA, Dale M, Dugourd A, et al. (June 2021)."Macrophage beta2-adrenergic receptor is dispensable for the adipose tissue inflammation and function".Molecular Metabolism.48:101220.doi:10.1016/j.molmet.2021.101220.PMC8086137.PMID33774223.
- ^Bolus WR, Gutierrez DA, Kennedy AJ, Anderson-Baucum EK, Hasty AH (October 2015)."CCR2 deficiency leads to increased eosinophils, alternative macrophage activation, and type 2 cytokine expression in adipose tissue".Journal of Leukocyte Biology.98(4): 467–477.doi:10.1189/jlb.3HI0115-018R.PMC4763864.PMID25934927.
- ^Boutens L, Stienstra R (May 2016)."Adipose tissue macrophages: going off track during obesity".Diabetologia.59(5): 879–894.doi:10.1007/s00125-016-3904-9.PMC4826424.PMID26940592.
- ^Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. (November 2005)."Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans".Journal of Lipid Research.46(11): 2347–2355.doi:10.1194/jlr.M500294-JLR200.PMID16150820.
- ^Kühl AA, Erben U, Kredel LI, Siegmund B (7 December 2015)."Diversity of Intestinal Macrophages in Inflammatory Bowel Diseases".Frontiers in Immunology.6:613.doi:10.3389/fimmu.2015.00613.PMC4670857.PMID26697009.
- ^abcSmythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, et al. (January 2005)."Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity".The Journal of Clinical Investigation.115(1): 66–75.doi:10.1172/JCI19229.PMC539188.PMID15630445.
- ^abMowat AM, Bain CC (2011)."Mucosal macrophages in intestinal homeostasis and inflammation".Journal of Innate Immunity.3(6): 550–564.doi:10.1159/000329099.PMC3224516.PMID22025201.
- ^Bain CC, Mowat AM (July 2014)."Macrophages in intestinal homeostasis and inflammation".Immunological Reviews.260(1): 102–117.doi:10.1111/imr.12192.PMC4141699.PMID24942685.
- ^Weiss G (January 2023). "Macrophage vesicles starve bacteria of iron".Nature Metabolism.5(1): 10–12.doi:10.1038/s42255-022-00719-1.PMID36658401.S2CID256030791.
- ^Epelman S, Lavine KJ, Randolph GJ (July 2014)."Origin and functions of tissue macrophages".Immunity.41(1): 21–35.doi:10.1016/j.immuni.2014.06.013.PMC4470379.PMID25035951.
- ^Mass E, Lachmann N (September 2021)."From macrophage biology to macrophage-based cellular immunotherapies".Gene Therapy.28(9): 473–476.doi:10.1038/s41434-021-00221-5.PMC8455330.PMID33542457.
- ^Epelman S, Lavine KJ, Randolph GJ (July 2014)."Origin and functions of tissue macrophages".Immunity.41(1): 21–35.doi:10.1016/j.immuni.2014.06.013.PMC4470379.PMID25035951.
- ^Italiani P, Boraschi D (August 2015)."New Insights Into Tissue Macrophages: From Their Origin to the Development of Memory".Immune Network.15(4): 167–176.doi:10.4110/in.2015.15.4.167.PMC4553254.PMID26330802.
- ^Lazarov T, Juarez-Carreño S, Cox N, Geissmann F (June 2023)."Physiology and diseases of tissue-resident macrophages".Nature.618(7966): 698–707.Bibcode:2023Natur.618..698L.doi:10.1038/s41586-023-06002-x.PMC10649266.PMID37344646.