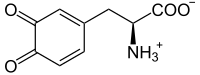
Melanin
| Melanin | |
|---|---|
 One possible structure of Eumelanin | |
| Material type | Heterogeneous biopolymer |

Melanin(/ˈmɛlənɪn/;fromAncient Greekμέλας(mélas)'black, dark') is a family ofbiomoleculesorganized asoligomersorpolymers,which among other functions provide thepigmentsof manyorganisms.[1]Melanin pigments are produced in a specialized group of cells known asmelanocytes.
There are five basic types of melanin:eumelanin,pheomelanin,neuromelanin,allomelaninandpyomelanin.[2]Eumelanin is produced through a multistage chemical process known asmelanogenesis,where theoxidationof theamino acidtyrosineis followed bypolymerization.Eumelanin is the most common type. Pheomelanin, which is produced when melanocytes are malfunctioning due to derivation of the gene to its recessive format, is acysteine-derivative that contains polybenzothiazineportions that are largely responsible for theredoryellowtint given to some skin or hair colors. Neuromelanin is found in the brain. Research has been undertaken to investigate its efficacy in treating neurodegenerative disorders such asParkinson's.[3]Allomelanin and pyomelanin are two types of nitrogen-free melanin.
In the human skin, melanogenesis is initiated by exposure toUV radiation,causing the skin to darken. Eumelanin is an effective absorbent of light; the pigment is able to dissipate over 99.9% of absorbed UV radiation.[4]Because of this property, eumelanin is thought to protect skin cells from UVA and UVB radiation damage, reducing the risk of folate depletion and dermal degradation. Exposure to UV radiation is associated with increased risk ofmalignant melanoma,a cancer of melanocytes (melanin cells). Studies have shown a lower incidence for skin cancer in individuals with more concentrated melanin, i.e. darkerskin tone.[5]
Melanin types[edit]
Eumelanin[edit]

Eumelanin has two forms linked to5,6-dihydroxyindole(DHI) and5,6-dihydroxyindole-2-carboxylic acid(DHICA). DHI-derived eumelanin is dark brown or black and insoluble, and DHICA -derived eumelanin which is lighter and soluble in alkali. Both eumelanins arise from the oxidation of tyrosine in specialized organelles calledmelanosomes.This reaction is catalyzed by the enzymetyrosinase.The initial product,dopaquinonecan transform into either 5,6-dihydroxyindole (DHI) or 5,6-dihydroxyindole-2-carboxylic acid (DHICA). DHI and DHICA are oxidized and then polymerize to form the two eumelanins.[6]
In natural conditions, DHI and DHICA often co-polymerize, resulting in a range of eumelanin polymers. These polymers contribute to the variety of melanin components in human skin and hair, ranging from light yellow/red pheomelanin to light brown DHICA-enriched eumelanin and dark brown or black DHI-enriched eumelanin. These final polymers differ in solubility and color.[6]
Analysis of highly pigmented (Fitzpatrick typeV and VI) skin finds that DHI-eumelanin comprises the largest portion, approximately 60–70%, followed by DHICA-eumelanin at 25–35%, and pheomelanin only 2–8%. Notably, while an enrichment of DHI-eumelanin occurs in duringsun tanning,it is accompanied by a decrease in DHICA-eumelanin and pheomelanin.[6]A small amount of black eumelanin in the absence of other pigments causes grey hair. A small amount of eumelanin in the absence of other pigments causes blond hair.[7]Eumelanin is present in the skin and hair, etc.
Pheomelanin[edit]

Pheomelanins (or phaeomelanins) impart a range of yellowish to reddish colors.[8]Pheomelanins are particularly concentrated in the lips, nipples, glans of the penis, and vagina.[9]When a small amount of eumelanin in hair (which would otherwise cause blond hair) is mixed with pheomelanin, the result is orange hair, which is typically called"red" or "ginger" hair.Pheomelanin is also present in the skin, and redheads consequently often have a more pinkish hue to their skin as well. Exposure of the skin to ultraviolet light increases pheomelanin content, as it does for eumelanin; but rather than absorbing light, pheomelanin within the hair and skin reflect yellow to red light, which may increase damage from UV radiation exposure.[10]
Pheomelanin production is highly dependent oncysteineavailability, which is transported into the melanosome, reacting with dopaquinone to form cys-dopa. Cys-dopa then undergoes several transformations before forming pheomelanin.[6]In chemical terms, pheomelanins differ from eumelanins in that the oligomer structure incorporatesbenzothiazineandbenzothiazoleunits that are produced,[11]instead of DHI andDHICA,when the amino acidL-cysteineis present.
Neuromelanin[edit]
Neuromelanin (NM) is an insoluble polymer pigment produced in specific populations ofcatecholaminergic neuronsin the brain. Humans have the largest amount of NM, which is present in lesser amounts in other primates, and totally absent in many other species.[12]The biological function remains unknown, although human NM has been shown to efficiently bindtransition metalssuch as iron, as well as other potentially toxic molecules. Therefore, it may play crucial roles inapoptosisand the relatedParkinson's disease.[13]
Other forms of melanins[edit]
Up until the 1960s, melanin was classified into eumelanin and pheomelanin. However in 1955 a melanin associated with nerve cells was discovered, neuromelanin. In 1972 a water-soluble form, pyomelanin was discovered. In 1976, allomelanin, the fifth form of the melanins was found in nature.[2]
Selenomelanin[edit]
It is possible to enrich melanin withseleniuminstead ofsulphur.This selenium analogue of pheomelanin has been successfully synthesized through chemical and biosynthetic routes using selenocystine as a feedstock.[14]Due selenium's higher atomic number, the obtained selenomelanin can be expected to provide better protection against ionising radiation as compared to the other known forms of melanin. This protection has been demonstrated with radiation experiments on human cells and bacteria, opening up the possibility of applications in space travel.[15]
Trichochromes[edit]
Trichochromes (formerly called trichosiderins) are pigments produced from the samemetabolic pathwayas the eumelanins and pheomelanins, but unlike those molecules they have low molecular weight. They occur in some red human hair.[16]
Humans[edit]

In humans, melanin is the primary determinant ofskin color.It is also found in hair, the pigmented tissue underlying theirisof the eye, and thestria vascularisof theinner ear.In the brain, tissues with melanin include themedullaand pigment-bearing neurons within areas of thebrainstem,such as thelocus coeruleus.It also occurs in thezona reticularisof theadrenal gland.[17]
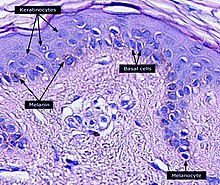
The melanin in the skin is produced bymelanocytes,which are found in thebasal layerof theepidermis.Although, in general, human beings possess a similar concentration of melanocytes in their skin, the melanocytes in some individuals and ethnic groups produce variable amounts of melanin. The ratio of eumelanin (74%) and pheomelanin (26%) in the epidermis is constant regardless of the degree of pigmentation.[18]Some humans have very little or no melanin synthesis in their bodies, a condition known asalbinism.[19]
Because melanin is an aggregate of smaller component molecules, there are many different types of melanin with different proportions and bonding patterns of these component molecules. Both pheomelanin and eumelanin are found in human skin and hair, but eumelanin is the most abundant melanin in humans, as well as the form most likely to be deficient in albinism.[20]
Other organisms[edit]
Melanins have very diverse roles and functions in various organisms. A form of melanin makes up the ink used by manycephalopods(seecephalopod ink) as a defense mechanism against predators. Melanins also protect microorganisms, such as bacteria and fungi, against stresses that involve cell damage such asUV radiationfrom the sun andreactive oxygen species.Melanin also protects against damage from high temperatures, chemical stresses (such asheavy metalsandoxidizing agents), and biochemical threats (such as host defenses against invading microbes).[21]Therefore, in many pathogenic microbes (for example, inCryptococcus neoformans,a fungus) melanins appear to play important roles invirulenceandpathogenicityby protecting the microbe againstimmuneresponses of itshost.In invertebrates, a major aspect of the innate immune defense system against invading pathogens involves melanin. Within minutes after infection, the microbe is encapsulated within melanin (melanization), and the generation of free radical byproducts during the formation of this capsule is thought to aid in killing them.[22]Some types of fungi, calledradiotrophic fungi,appear to be able to use melanin as aphotosynthetic pigmentthat enables them to capturegamma rays[23]and harness this energy for growth.[24]
Infish,melanin occurs not only in the skin but also in internal organs such as eyes. Most fish species use eumelanin,[25][26]butStegastes apicalisandCyprinus carpiouse pheomelanin instead.[27][28]
The darkerfeathersof birds owe their color to melanin and are less readily degraded by bacteria than unpigmented ones or those containingcarotenoidpigments.[29]Feathers that contain melanin are also 39% more resistant to abrasion than those that do not because melanin granules help fill the space between thekeratinstrands that form feathers.[30][31]Pheomelanin synthesis in birds implies the consumption of cysteine, a semi‐essential amino acid that is necessary for the synthesis of the antioxidant glutathione (GSH) but that may be toxic if in excess in the diet. Indeed, many carnivorous birds, which have a high protein content in their diet, exhibit pheomelanin‐based coloration.[32]
Melanin is also important inmammalianpigmentation.[33]The coat pattern of mammals is determined by theagouti genewhich regulates the distribution of melanin.[34][35]The mechanisms of the gene have been extensively studied in mice to provide an insight into the diversity of mammalian coat patterns.[36]
Melanin inarthropodshas been observed to be deposited in layers thus producing aBragg reflectorof alternating refractive index. When the scale of this pattern matches the wavelength of visible light,structural colorationarises: giving a number of species aniridescentcolor.[37][38]
Arachnidsare one of the few groups in which melanin has not been easily detected, though researchers found data suggesting spiders do in fact produce melanin.[39]
Some moth species, including thewood tiger moth,convert resources to melanin to enhance their thermoregulation. As the wood tiger moth has populations over a large range of latitudes, it has been observed that more northern populations showed higher rates of melanization. In both yellow and white male phenotypes of the wood tiger moth, individuals with more melanin had a heightened ability to trap heat but an increased predation rate due to a weaker and less effectiveaposematicsignal.[40]
Melanin protectsDrosophilaflies andmiceagainst DNA damage from non-UV radiation.[41]Important studies in Drosophila models include Hopwoodet al.,1985.[41]Much of our understanding of theradioprotectiveeffects of melanin againstgamma radiationcome from the laboratories and research groups of Irma Mosse.[42][43][44][45][46][47][48]: 1151 Mosse began inradiobiologyin the Soviet era, was increasingly supported by government funding in the wake of the discovery ofradiotrophicmicrobes inChernobyl,and as of 2022[update]continues under theBelarusian Institute of Genetics and Cytology.[47]Her most significant contribution is Mosseet al.,2000 on mice[42][43][44][45][46][47][48]: 1151 but also includes Mosseet al.,1994,[46]Mosseet al.,1997,[46]Mosseet al.,1998,[45]Mosseet al.,2001,[46]Mosseet al.,2002,[45][46]Mosseet al.,2006,[45][46]Mosseet al.,2007[46]and Mosseet al.,2008.[46]
Plants[edit]

Melanin produced by plants are sometimes referred to as 'catechol melanins' as they can yieldcatecholon alkali fusion. It is commonly seen in theenzymatic browningof fruits such as bananas. Chestnut shell melanin can be used as an antioxidant and coloring agent.[49]Biosynthesis involves the oxidation ofindole-5,6-quinoneby the tyrosinase typepolyphenol oxidasefromtyrosineandcatecholaminesleading to the formation of catechol melanin. Despite this many plants contain compounds which inhibit the production of melanins.[50]
Interpretation as a single monomer[edit]
It is now understood that melanins do not have a single structure or stoichiometry.[citation needed]Nonetheless, chemical databases such as PubChem include structural and empirical formulae; typically3,8-Dimethyl-2,7-dihydrobenzo[1,2,3-cd:4,5,6-c′d′]diindole-4,5,9,10-tetrone.This can be thought of as a single monomer that accounts for the measured elemental composition and some properties of melanin, but is unlikely to be found in nature.[51]Solano[51]claims that this misleading trend stems from a report of an empirical formula in 1948,[52]but provides no other historical detail.
![3,8-Dimethyl-2,7-dihydrobenzo[1,2,3-cd:4,5,6-c′d′]diindole-4,5,9,10-tetrone](https://upload.wikimedia.org/wikipedia/commons/thumb/2/20/Melanin.svg/240px-Melanin.svg.png)
| |
![3,8-Dimethyl-2,7-dihydrobenzo[1,2,3-cd:4,5,6-c′d′]diindole-4,5,9,10-tetrone ball and stick model](https://upload.wikimedia.org/wikipedia/commons/thumb/7/7b/Melanin_ball_and_stick.png/240px-Melanin_ball_and_stick.png)
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,8-Dimethyl-2,7-dihydrobenzo[1,2,3-cd:4,5,6-c′d′]diindole-4,5,9,10-tetrone | |
| Identifiers | |
| ChemSpider | |
PubChemCID
|
|
| Properties | |
| C18H10N2O4 | |
| Molar mass | 318.288g·mol−1 |
| Density | 1.6 to 1.8 g/cm3 |
| Melting point | < −20 °C (−4 °F; 253 K) |
| Boiling point | 450 to 550 °C (842 to 1,022 °F; 723 to 823 K) |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Biosynthetic pathways[edit]
The first step of the biosynthetic pathway for both eumelanins and pheomelanins iscatalysedbytyrosinase.[53]
Dopaquinone can combine withcysteineby two pathways to benzothiazines and pheomelanins
- dopaquinone + cysteine → 5-S-cysteinyldopa → benzothiazine intermediate → pheomelanin
- dopaquinone + cysteine → 2-S-cysteinyldopa → benzothiazine intermediate → pheomelanin
Also, dopaquinone can be converted toleucodopachromeand follow two more pathways to the eumelanins
- dopaquinone → leucodopachrome →dopachrome→ 5,6-dihydroxyindole-2-carboxylic acid → quinone → eumelanin
- dopaquinone → leucodopachrome → dopachrome → 5,6-dihydroxyindole →quinone→ eumelanin
Detailed metabolic pathways can be found in theKEGGdatabase (seeExternal links).
-
L-tyrosine
-
L-DOPA
-
L-dopaquinone
-
L-leucodopachrome
-
L-dopachrome
Microscopic appearance[edit]
Melanin is brown, non-refractile, and finely granular with individual granules having a diameter of less than 800 nanometers. This differentiates melanin from commonblood breakdown pigments,which are larger, chunky, and refractile, and range in color from green to yellow or red-brown. In heavily pigmented lesions, dense aggregates of melanin can obscure histologic detail. A dilute solution ofpotassium permanganateis an effective melanin bleach.[54]
Genetic disorders and disease states[edit]
There are approximately nine types ofoculocutaneous albinism,which is mostly an autosomal recessive disorder. Certain ethnicities have higher incidences of different forms. For example, the most common type, called oculocutaneous albinism type 2 (OCA2), is especially frequent among people of black African descent and white Europeans. People with OCA2 usually have fair skin, but are often not as pale as OCA1. They (OCA2 or OCA1? see comments in History) have pale blonde to golden, strawberry blonde, or even brown hair, and most commonly blue eyes. 98.7–100% of modern Europeans are carriers of the derived alleleSLC24A5,a known cause of nonsyndromic oculocutaneous albinism. It is an autosomal recessive disorder characterized by acongenitalreduction or absence of melanin pigment in the skin, hair, and eyes. The estimated frequency of OCA2 among African-Americans is 1 in 10,000, which contrasts with a frequency of 1 in 36,000 in white Americans.[55]In some African nations, the frequency of the disorder is even higher, ranging from 1 in 2,000 to 1 in 5,000.[56]Another form of Albinism, the "yellow oculocutaneous albinism", appears to be more prevalent among theAmish,who are of primarily Swiss and German ancestry. People with this IB variant of the disorder commonly have white hair and skin at birth, but rapidly develop normal skin pigmentation in infancy.[56]
Ocular albinism affects not only eye pigmentation but visual acuity, as well. People with albinism typically test poorly, within the 20/60 to 20/400 range. In addition, two forms of albinism, with approximately 1 in 2,700 most prevalent among people of Puerto Rican origin, are associated with mortality beyond melanoma-related deaths.
The connection between albinism anddeafnessis well known, though poorly understood. In his 1859 treatiseOn the Origin of Species,Charles Darwinobserved that "cats which are entirely white and have blue eyes are generally deaf".[57]In humans, hypopigmentation and deafness occur together in the rareWaardenburg's syndrome,predominantly observed among theHopiin North America.[58]The incidence of albinism in Hopi Indians has been estimated as approximately 1 in 200 individuals. Similar patterns of albinism and deafness have been found in other mammals, including dogs and rodents. However, a lack of melaninper sedoes not appear to be directly responsible for deafness associated with hypopigmentation, as most individuals lacking the enzymes required to synthesize melanin have normal auditory function.[59]Instead, the absence ofmelanocytesin the stria vascularis of the inner ear results incochlearimpairment,[60]though the reasons for this are not fully understood.
In Parkinson's disease, a disorder that affectsneuromotorfunctioning, there is decreased neuromelanin in the substantia nigra and locus coeruleus as a consequence of specific dropping out of dopaminergic and noradrenergic pigmented neurons. This results in diminisheddopamineandnorepinephrinesynthesis. While no correlation between race and the level of neuromelanin in the substantia nigra has been reported, the significantly lower incidence of Parkinson's in blacks than in whites has "prompt[ed] some to suggest that cutaneous melanin might somehow serve to protect the neuromelanin in substantia nigra from external toxins."[61]
In addition to melanin deficiency, the molecular weight of the melanin polymer may be decreased by various factors such as oxidative stress, exposure to light, perturbation in its association with melanosomalmatrix proteins,changes inpH,or in local concentrations of metal ions. A decreased molecular weight or a decrease in the degree of polymerization ofocular melaninhas been proposed to turn the normally anti-oxidant polymer into apro-oxidant.In its pro-oxidant state, melanin has been suggested to be involved in the causation and progression ofmacular degenerationandmelanoma.[62]Rasagiline,an important monotherapy drug in Parkinson's disease, has melanin binding properties, and melanoma tumor reducing properties.[63]
Higher eumelanin levels also can be a disadvantage, however, beyond a higher disposition toward vitamin D deficiency. Dark skin is a complicating factor in the laser removal ofport-wine stains.Effective in treating white skin, in general, lasers are less successful in removing port-wine stains in people of Asian or African descent. Higher concentrations of melanin in darker-skinned individuals simply diffuse and absorb the laser radiation, inhibiting light absorption by the targeted tissue. In a similar manner, melanin can complicate laser treatment of other dermatological conditions in people with darker skin.
Frecklesandmolesare formed where there is a localized concentration of melanin in the skin. They are highly associated with pale skin.
Nicotinehas an affinity for melanin-containing tissues because of its precursor function in melanin synthesis or its irreversible binding of melanin. This has been suggested to underlie the increasednicotine dependenceand lowersmoking cessationrates in darker pigmented individuals.[64]
Human adaptations[edit]
Physiology[edit]
Melanocytes insert granules of melanin into specialized cellularvesiclescalledmelanosomes.These are then transferred into thekeratinocytecells of the humanepidermis.The melanosomes in each recipient cell accumulate atop thecell nucleus,where they protect the nuclearDNAfrom mutations caused by theionizing radiationof the sun'sultravioletrays. In general, people whose ancestors lived for long periods in the regions of the globe near theequatorhave larger quantities of eumelanin in their skins. This makes their skins brown or black and protects them against high levels of exposure to the sun, which more frequently result inmelanomasin lighter-skinned people.[65]
Not all the effects of pigmentation are advantageous. Pigmentation increases the heat load in hot climates, and dark-skinned people absorb 30% more heat from sunlight than do very light-skinned people, although this factor may be offset by more profuse sweating. In cold climates dark skin entails more heat loss by radiation. Pigmentation also hinders synthesis ofvitamin D.Since pigmentation appears to be not entirely advantageous to life in the tropics, other hypotheses about its biological significance have been advanced; for example a secondary phenomenon induced by adaptation to parasites and tropical diseases.[66]
Evolutionary origins[edit]
Early humansevolved dark skin color, as an adaptation to a loss of body hair that increased the effects of UV radiation. Before the development of hairlessness, early humans might have had light skin underneath their fur, similar to that found in otherprimates.[67]Anatomically modern humansevolved in Africa between 200,000 and 100,000 years ago,[68]and then populated the rest of the world through migration between 80,000 and 50,000 years ago, in some areasinterbreedingwith certainarchaic humanspecies (Neanderthals,Denisovans,and possibly others).[69]The first modern humans had darker skin as the indigenous people of Africa today. Following migration and settlement in Asia and Europe, the selective pressure dark UV-radiation protecting skin decreased where radiation from the sun was less intense. This resulted in the current range of human skin color. Of the two common gene variants known to be associated with pale human skin,Mc1rdoes not appear to have undergone positive selection,[70]whileSLC24A5has undergone positive selection.[71]
Effects[edit]
As with peoples having migrated northward, those with light skin migrating toward the equator acclimatize to the much stronger solar radiation. Nature selects for less melanin when ultraviolet radiation is weak. Most people's skin darkens when exposed to UV light, giving them more protection when it is needed. This is the physiological purpose ofsun tanning.Dark-skinned people, who produce more skin-protecting eumelanin, have a greater protection againstsunburnand the development of melanoma, a potentially deadly form of skin cancer, as well as other health problems related to exposure to strongsolar radiation,including thephotodegradationof certainvitaminssuch asriboflavins,carotenoids,tocopherol,andfolate.[72]
Melanin in the eyes, in theirisandchoroid,helps protect fromultravioletandhigh-frequency visible light;people withblue, green, and grey eyesare more at risk of sun-related eye problems. Furthermore, the ocular lens yellows with age, providing added protection. However, the lens also becomes more rigid with age, losing most of itsaccommodation—the ability to change shape to focus from far to near—a detriment due probably toproteincrosslinking caused by UV exposure.
Recent research suggests that melanin may serve a protective role other than photoprotection.[73]Melanin is able to effectivelychelatemetal ions through its carboxylate and phenolic hydroxyl groups, often much more efficiently than the powerful chelating ligand ethylenediaminetetraacetate (EDTA). Thus, it may serve to sequester potentially toxic metal ions, protecting the rest of the cell. This hypothesis is supported by the fact that the loss of neuromelanin, observed in Parkinson's disease, is accompanied by an increase in iron levels in the brain.
Physical properties and technological applications[edit]
Evidence exists for a highly cross-linkedheteropolymerboundcovalentlyto matrix scaffoldingmelanoproteins.[74]It has been proposed that the ability of melanin to act as anantioxidantis directly proportional to its degree of polymerization ormolecular weight.[75]Suboptimal conditions for the effective polymerization of melaninmonomersmay lead to formation of pro-oxidant melanin with lower-molecular-weight, implicated in the causation and progression ofmacular degenerationandmelanoma.[76]Signaling pathwaysthatupregulatemelanization in theretinal pigment epithelium(RPE) also may be implicated in thedownregulationofrodouter segmentphagocytosisby the RPE. This phenomenon has been attributed in part tofovealsparing inmacular degeneration.[77]
Role in melanoma metastasis[edit]
Heavily pigmented melanoma cells have aYoung's modulusof about 4.93 kPa, compared to non-pigmented cells, with a value of 0.98 kPa.[78]Theelasticityof melanoma cells is crucial to metastasis and growth; non-pigmented tumors were larger than pigmented tumors, and spread far more easily. Pigmented and non-pigmented cells are both present in melanomatumors,so that they can both bedrug-resistantand metastatic.[78]
See also[edit]
- Albino
- Albinism in biology
- Griscelli syndrome,a syndrome characterised by hypopigmentation
- Human skin color
- Melanin theory
- Melanism
- Melanogenesis,melanin production
- Risks and benefits of sun exposure
- Skin whitening
- Vitamin D
References[edit]
- ^Casadevall, Arturo (2018). "Melanin triggers antifungal defences".Nature.555(7696): 319–320.Bibcode:2018Natur.555..319C.doi:10.1038/d41586-018-02370-x.ISSN0028-0836.PMID29542711.S2CID3832753.
- ^abCao, Wei; Zhou, Xuhao; McCallum, Naneki C.; Hu, Ziying; Ni, Qing Zhe; Kapoor, Utkarsh; Heil, Christian M.; Cay, Kristine S.; Zand, Tara; Mantanona, Alex J.; Jayaraman, Arthi (9 February 2021)."Unraveling the Structure and Function of Melanin through Synthesis".Journal of the American Chemical Society.143(7): 2622–2637.doi:10.1021/jacs.0c12322.hdl:1854/LU-8699336.ISSN0002-7863.PMID33560127.S2CID231872855.
- ^Haining, Robert L.; Achat-Mendes, Cindy (March 2017)."Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator".Neural Regeneration Research.12(3): 372–375.doi:10.4103/1673-5374.202928.PMC5399705.PMID28469642.
- ^Meredith P, Riesz J (2004). "Radiative relaxation quantum yields for synthetic eumelanin".Photochemistry and Photobiology.79(2): 211–6.arXiv:cond-mat/0312277.doi:10.1111/j.1751-1097.2004.tb00012.x.PMID15068035.S2CID222101966.
- ^Brenner M, Hearing VJ (2008)."The protective role of melanin against UV damage in human skin".Photochemistry and Photobiology.84(3): 539–49.doi:10.1111/j.1751-1097.2007.00226.x.PMC2671032.PMID18435612.
- ^abcdAlaluf, Simon; Heath, Alan; Carter, Nik; Atkins, Derek; Mahalingam, Harish; Barrett, Karen; Kolb, Ria; Smit, Nico (2001). "Variation in Melanin Content and Composition in Type V and VI Photoexposed and Photoprotected Human Skin: The Dominant Role of DHI".Pigment Cell Research.14(5): 337–347.doi:10.1034/j.1600-0749.2001.140505.x.ISSN0893-5785.PMID11601655.
- ^Ito, S.; Wakamatsu, K. (December 2011). "Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin".Journal of the European Academy of Dermatology and Venereology.25(12): 1369–1380.doi:10.1111/j.1468-3083.2011.04278.x.ISSN1468-3083.PMID22077870.S2CID5121042.
- ^Slominski A, Tobin DJ, Shibahara S, Wortsman J (October 2004). "Melanin pigmentation in mammalian skin and its hormonal regulation".Physiological Reviews.84(4): 1155–228.doi:10.1152/physrev.00044.2003.PMID15383650.S2CID21168932.
- ^"pheomelanin".MetaCyc Metabolic Pathway Database.2010.[full citation needed]
- ^Thody, A. J.; Higgins, E. M.; Wakamatsu, K.; Ito, S.; Burchill, S. A.; Marks, J. M. (August 1991)."Pheomelanin as well as eumelanin is present in human epidermis".The Journal of Investigative Dermatology.97(2): 340–344.doi:10.1111/1523-1747.ep12480680.PMID2071942.
- ^Greco G, Panzella L, Verotta L, d'Ischia M, Napolitano A (April 2011). "Uncovering the structure of human red hair pheomelanin: benzothiazolylthiazinodihydroisoquinolines as key building blocks".Journal of Natural Products.74(4): 675–82.doi:10.1021/np100740n.PMID21341762.
- ^Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL (2005). "Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease".Prog Neurobiol.75(2): 109–124.doi:10.1016/j.pneurobio.2005.02.001.PMID15784302.S2CID503902.
- ^Double KL (2006). "Functional effects of neuromelanin and synthetic melanin in model systems".J Neural Transm.113(6): 751–756.doi:10.1007/s00702-006-0450-5.PMID16755379.S2CID23096297.
- ^Wei Cao; et al. (2020). "Selenomelanin: An Abiotic Selenium Analogue of Pheomelanin".Journal of the American Chemical Society.142(29): 12802–12810.doi:10.1021/jacs.0c05573.PMID32638590.S2CID220413025.
- ^Mark Heiden (8 July 2020)."New biomaterial could shield against harmful radiation".Northwestern University.Retrieved29 January2023.
- ^Prota, G.; Searle, A. G. (1978)."Biochemical sites of gene action for melanogenesis in mammals".Annales de Génétique et de Sélection Animale.10(1): 1–8.doi:10.1186/1297-9686-10-1-1.PMC2757330.PMID22896083.
- ^Solano, F. (2014)."Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes".New Journal of Science.2014:1–28.doi:10.1155/2014/498276.
- ^Del Bino, Sandra; Ito, Shosuke; Sok, Juliette; Wakamatsu, Kazumasa (2022)."5,6‐Dihydroxyindole eumelanin content in human skin with varying degrees of constitutive pigmentation".Pigment Cell & Melanoma Research.35(6): 622–626.doi:10.1111/pcmr.13062.ISSN1755-1471.PMC9804219.PMID35933709.
- ^Cichorek, Mirosława; Wachulska, Małgorzata; Stasiewicz, Aneta; Tymińska, Agata (20 February 2013)."Skin melanocytes: biology and development".Advances in Dermatology and Allergology.30(1): 30–41.doi:10.5114/pdia.2013.33376.PMC3834696.PMID24278043.
- ^"oculocutaneous albinism".Genetics Home Reference.Retrieved25 September2017.
- ^Hamilton AJ, Gomez BL (March 2002)."Melanins in fungal pathogens".Journal of Medical Microbiology.51(3): 189–91.doi:10.1099/0022-1317-51-3-189.PMID11871612.
- ^Cerenius L, Söderhäll K (April 2004). "The prophenoloxidase-activating system in invertebrates".Immunological Reviews.198:116–26.doi:10.1111/j.0105-2896.2004.00116.x.PMID15199959.S2CID10614298.
- ^Castelvecchi, Davide (26 May 2007). "Dark Power: Pigment seems to put radiation to good use".Science News.171(21): 325.doi:10.1002/scin.2007.5591712106.
- ^Dadachova E, Bryan RA, Huang X, et al. (2007)."Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi".PLOS ONE.2(5): e457.Bibcode:2007PLoSO...2..457D.doi:10.1371/journal.pone.0000457.PMC1866175.PMID17520016.
- ^Sköld HN, Aspengren S, Cheney KL, Wallin M (2016). "Chapter Four - Fish Chromatophores—From Molecular Motors to Animal Behavior".International Review of Cell and Molecular Biology.321:171–219.doi:10.1016/bs.ircmb.2015.09.005.PMID26811288.
- ^Kottler, Verena A.; Künstner, Axel; Schartl, Manfred (May 2015)."Pheomelanin in fish?".Pigment Cell & Melanoma Research.28(3): 355–356.doi:10.1111/pcmr.12359.ISSN1755-1471.PMID25660115.S2CID8877527.
- ^Xu, Peng; Zhang, Xiaofeng; Wang, Xumin; Li, Jiongtang; Liu, Guiming; Kuang, Youyi; Xu, Jian; Zheng, Xianhu; Ren, Lufeng; Wang, Guoliang; Zhang, Yan; Huo, Linhe; Zhao, Zixia; Cao, Dingchen; Lu, Cuiyun (November 2014)."Genome sequence and genetic diversity of the common carp, Cyprinus carpio".Nature Genetics.46(11): 1212–1219.doi:10.1038/ng.3098.ISSN1061-4036.PMID25240282.
- ^Mouchet SR, Cortesi F, Bokic B, Lazovic V, Vukusic P, Marshall NJ, Kolaric B (1 November 2023)."Morphological and Optical Modification of Melanosomes in Fish Integuments upon Oxidation".Optics.4(4): 563–562.doi:10.3390/opt4040041.
- ^Gunderson, Alex R.; Frame, Alicia M.; Swaddle, John P.; Forsyth, Mark H. (1 September 2008). "Resistance of melanized feathers to bacterial degradation: is it really so black and white?".Journal of Avian Biology.39(5): 539–545.doi:10.1111/j.0908-8857.2008.04413.x.
- ^Bonser, Richard H. C. (1995)."Melanin and the Abrasion Resistance of Feathers".Condor.97(2): 590–591.doi:10.2307/1369048.JSTOR1369048.
- ^Galván, Ismael; Solano, Francisco (8 April 2016)."Bird Integumentary Melanins: Biosynthesis, Forms, Function and Evolution".International Journal of Molecular Sciences.17(4): 520.doi:10.3390/ijms17040520.PMC4848976.PMID27070583.
- ^Rodríguez-Martínez, Sol; Galván, Ismael (2020). "Juvenile pheomelanin-based plumage coloration has evolved more frequently in carnivorous species".Ibis.162(1): 238–244.doi:10.1111/ibi.12770.hdl:10261/207451.ISSN1474-919X.S2CID202018215.
- ^Jimbow, K; Quevedo WC, Jr; Fitzpatrick, TB; Szabo, G (July 1976)."Some aspects of melanin biology: 1950–1975".The Journal of Investigative Dermatology.67(1): 72–89.doi:10.1111/1523-1747.ep12512500.PMID819593.
- ^Meneely, Philip (2014).Genetic Analysis: Genes, Genomes, and Networks in Eukaryotes.Oxford University Press.ISBN9780199681266.
- ^Griffiths, Anthony JF; Miller, Jeffrey H.; Suzuki, David T.; Lewontin, Richard C.; Gelbart, William M. (2000)."Gene interaction in coat color of mammals".NCBI.[permanent dead link]
- ^Millar, S. E.; Miller, M. W.; Stevens, M. E.; Barsh, G. S. (October 1995). "Expression and transgenic studies of the mouse agouti gene provide insight into the mechanisms by which mammalian coat color patterns are generated".Development.121(10): 3223–3232.doi:10.1242/dev.121.10.3223.PMID7588057.
- ^Neville, A. C. (2012).Biology of the Arthropod Cuticle.Springer Science & Business Media.ISBN9783642809101.
- ^Mouchet, Sébastien R; Deparis, Olivier (2021),Natural Photonics and Bioinspiration(1st ed.), Artech House,ISBN978-163-081-797-8
- ^Hsiung, B.-K.; Blackledge, T. A.; Shawkey, M. D. (2015)."Spiders do have melanin after all".Journal of Experimental Biology.218(22): 3632–3635.doi:10.1242/jeb.128801.PMID26449977.
- ^Hegna, Robert H.; Nokelainen, Ossi; Hegna, Jonathan R.; Mappes, Johanna (2013)."To quiver or to shiver: increased melanization benefits thermoregulation, but reduces warning signal efficacy in the wood tiger moth".Proc. R. Soc. B.280(1755): 20122812.doi:10.1098/rspb.2012.2812.PMC3574392.PMID23363631.
- ^abMosse, Irma B.; Dubovic, Boris V.; Plotnikova, Svetlana I.; Kostrova, Ludmila N.; Molophei, Vadim; Subbot, Svetlana T.; Maksimenya, Inna P. (20–25 May 2001). Obelic, B.; Ranogajev-Komor, M.; Miljanic, S.; Krajcar Bronic, I. (eds.).Melanin is Effective Radioprotector against Chronic Irradiation and Low Radiation Doses.IRPA Regional Congress on Radiation Protection in Central Europe: Radiation Protection and Health.INIS.Dubrovnik(Croatia):Croatian Radiation Protection Association.p. 35 (of 268).
- ^abGessler, N. N.; Egorova, A. S.; Belozerskaya, T. A. (2014)."Melanin pigments of fungi under extreme environmental conditions (Review)".Applied Biochemistry and Microbiology.50(2).Pleiades Publishing:105–113.doi:10.1134/s0003683814020094.ISSN0003-6838.PMID25272728.S2CID8570835.
- ^abNenoi, M; Wang, B; Vares, G (12 June 2014)."In vivo radioadaptive response".Toxicology.34(3).Sage:272–283.doi:10.1177/0960327114537537.ISSN0960-3271.PMC4442823.PMID24925363.
- ^abLiu, Heng; Yang, Youyuan; Liu, Yu; Pan, Jingjing; Wang, Junqing; Man, Fengyuan; Zhang, Weiguo; Liu, Gang (7 February 2020)."Melanin-Like Nanomaterials for Advanced Biomedical Applications: A Versatile Platform with Extraordinary Promise".Advanced Science.7(7).Wiley-VCH:1903129.doi:10.1002/advs.201903129.ISSN2198-3844.PMC7141020.PMID32274309.
- ^abcdeMosse, Irma B. (2012). "Genetic effects of ionizing radiation – some questions with no answers".Journal of Environmental Radioactivity.112.Elsevier:70–75.Bibcode:2012JEnvR.112...70M.doi:10.1016/j.jenvrad.2012.05.009.ISSN0265-931X.PMID22683898.
- ^abcdefghiMosse, Irma; Kilchevsky, Alexander; Nikolova, Nevena; Zhelev, Nikolai (14 December 2016)."Some problems and errors in cytogenetic biodosimetry".Biotechnology & Biotechnological Equipment.31(3).Taylor & Francis:460–468.doi:10.1080/13102818.2016.1259018.ISSN1310-2818.S2CID59398089.
- ^abcMosse, Irma (18 January 2022). "Radiobiology in my life – Irma Mosse".International Journal of Radiation Biology.98(3: Women in Radiobiology).Taylor & Francis:474–478.doi:10.1080/09553002.2022.2026517.ISSN0955-3002.PMID34994663.S2CID245823003.
- ^abDadachova, Ekaterina; Casadevall, Arturo (2011). Horikoshi, Kōki (ed.).Extremophiles handbook.Tokyo New York City:Springer.pp. xxix+1247.ISBN978-4-431-53898-1.OCLC700199222.ISBN978-4-431-53897-4.OCLC711778164.
- ^Yao, Zeng-Yu; Qi, Jian-Hua (22 April 2016)."Comparison of Antioxidant Activities of Melanin Fractions from Chestnut Shell".Molecules.21(4): 487.doi:10.3390/molecules21040487.PMC6273334.PMID27110763.
- ^Kim, Y.-J.; Uyama, H. (15 May 2005)."Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future".Cellular and Molecular Life Sciences.62(15): 1707–1723.doi:10.1007/s00018-005-5054-y.PMC11139184.PMID15968468.S2CID8280251.
- ^abSolano, F. (2014)."Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes".New Journal of Science.2014(498276): 1–28.doi:10.1155/2014/498276.
- ^Mason, H. S. (1948)."The chemistry of melanin. Mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase".Journal of Biological Chemistry.172(1): 83–99.doi:10.1016/S0021-9258(18)35614-X.PMID18920770.
- ^Zaidi, Kamal Uddin; Ali, Ayesha S.; Ali, Sharique A.; Naaz, Ishrat (2014)."Microbial Tyrosinases: Promising Enzymes for Pharmaceutical, Food Bioprocessing, and Environmental Industry".Biochemistry Research International.2014:1–16 (see Fig. 3).doi:10.1155/2014/854687.PMC4033337.PMID24895537.
- ^"Melanin".pubchem.ncbi.nlm.nih.gov.Retrieved25 September2017.
- ^"Oculocutaneous Albinism".Archived fromthe originalon 23 December 2008.
- ^abPeracha, Mohammed O.; Cosgrove, Frances M.; Garcia-Valenzuela, Enrique; Eliott, Dean (5 November 2015). Roy, Sr, Hampton; Talavera, Francisco; Rowsey, J. James (eds.)."Ocular Manifestations of Albinism: Background, Pathophysiology, Epidemiology".Medscape.Additional contributions from Kilbourn Gordon, III.WebMD.Archivedfrom the original on 28 March 2017.Retrieved8 September2022– viaeMedicine.
- ^"Causes of Variability".Archived fromthe originalon 23 September 2006.Retrieved18 September2006.
- ^EntrezGene300700
- ^EntrezGene606933
- ^Cable J, Huszar D, Jaenisch R, Steel KP (February 1994). "Effects of mutations at the W locus (c-kit) on inner ear pigmentation and function in the mouse".Pigment Cell Research.7(1): 17–32.doi:10.1111/j.1600-0749.1994.tb00015.x.PMID7521050.
- ^"Lewy Body Disease".Archived fromthe originalon 21 July 2009.
- ^Meyskens FL, Farmer P, Fruehauf JP (June 2001)."Redox regulation in human melanocytes and melanoma"(PDF).Pigment Cell Research.14(3): 148–54.doi:10.1034/j.1600-0749.2001.140303.x.PMID11434561.
- ^Meier-Davis SR, Dines K, Arjmand FM, et al. (December 2012). "Comparison of oral and transdermal administration of rasagiline mesylate on human melanoma tumor growth in vivo".Cutaneous and Ocular Toxicology.31(4): 312–7.doi:10.3109/15569527.2012.676119.PMID22515841.S2CID30344869.
- ^King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET (June 2009). "Link between facultative melanin and tobacco use among African Americans".Pharmacology Biochemistry and Behavior.92(4): 589–96.doi:10.1016/j.pbb.2009.02.011.PMID19268687.S2CID3070838.
- ^"Human Skin Color Variation".The Smithsonian Institution's Human Origins Program.20 June 2012.Retrieved24 August2019.
- ^Berth-Jones, J. (2010), "Constitutive pigmentation, human pigmentation and the response to sun exposure", in Tony Burns; Stephen Breathnach; Neil Cox; Christopher Griffiths (eds.),Rook's Textbook of Dermatology,vol. 3 (8th ed.), Wiley-Blackwell, p. 58.9,ISBN978-1-4051-6169-5
- ^Wade, Nicholas (19 August 2003)."Why Humans and Their Fur Parted Ways".The New York Times.ISSN0362-4331.Retrieved24 August2019.
- ^Tishkoff SA, Reed FA, Friedlaender FR, et al. (May 2009)."The genetic structure and history of Africans and African Americans".Science.324(5930): 1035–44.Bibcode:2009Sci...324.1035T.doi:10.1126/science.1172257.PMC2947357.PMID19407144.
- ^"A Single Migration From Africa Populated the World, Studies Find".The New York Times.22 September 2016.
- ^Harding RM, Healy E, Ray AJ, et al. (April 2000)."Evidence for variable selective pressures at MC1R".American Journal of Human Genetics.66(4): 1351–61.doi:10.1086/302863.PMC1288200.PMID10733465.
- ^Lamason RL, Mohideen MA, Mest JR, et al. (December 2005). "SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans".Science.310(5755): 1782–6.Bibcode:2005Sci...310.1782L.doi:10.1126/science.1116238.PMID16357253.S2CID2245002.
- ^Jablonski, Nina G.; Chaplin, George (11 May 2010)."Human skin pigmentation as an adaptation to UV radiation".Proceedings of the National Academy of Sciences.107(Supplement 2): 8962–8968.Bibcode:2010PNAS..107.8962J.doi:10.1073/pnas.0914628107.PMC3024016.PMID20445093.
- ^Liu Y, Hong L, Kempf VR, Wakamatsu K, Ito S, Simon JD (June 2004). "Ion-exchange and adsorption of Fe(III) by Sepia melanin".Pigment Cell Research.17(3): 262–9.doi:10.1111/j.1600-0749.2004.00140.x.PMID15140071.
- ^Donatien PD, Orlow SJ (August 1995). "Interaction of melanosomal proteins with melanin".European Journal of Biochemistry.232(1): 159–64.doi:10.1111/j.1432-1033.1995.tb20794.x.PMID7556145.
- ^Sarangarajan R, Apte SP (2005). "Melanin aggregation and polymerization: possible implications in age-related macular degeneration".Ophthalmic Research.37(3): 136–41.doi:10.1159/000085533.PMID15867475.S2CID27499198.
- ^Meyskens FL, Farmer PJ, Anton-Culver H (April 2004)."Etiologic pathogenesis of melanoma: a unifying hypothesis for the missing attributable risk"(PDF).Clinical Cancer Research.10(8): 2581–3.doi:10.1158/1078-0432.ccr-03-0638.PMID15102657.S2CID26079375.
- ^Sarangarajan R, Apte SP (2005). "Melanization and phagocytosis: implications for age related macular degeneration".Molecular Vision.11:482–90.PMID16030499.
- ^abSarna, Michal; Krzykawska-Serda, Martyna; Jakubowska, Monika; Zadlo, Andrzej; Urbanska, Krystyna (26 June 2019)."Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion".Scientific Reports.9(1): 9280.Bibcode:2019NatSR...9.9280S.doi:10.1038/s41598-019-45643-9.ISSN2045-2322.PMC6594928.PMID31243305.
External links[edit]
- "Absorption spectrum of melanin".Department of Computer Science and Technology.
- "Tyrosine metabolism—Reference pathway".Kyoto Encyclopedia of Genes and Genomes.Retrieved13 June2024.
- "Melanogenesis".Kyoto Encyclopedia of Genes and Genomes.Retrieved13 June2024.