Merimepodib
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
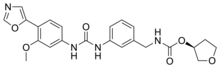
| Formula | C23H24N4O6 |
| Molar mass | 452.467g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Merimepodib(VX-497) is a drug which acts as an inhibitor of theenzymeinosine monophosphate dehydrogenase,which is required for the synthesis ofnucleotidebases containingguanine.This consequently inhibits synthesis of DNA and RNA, and results inantiviralandimmunosuppressiveeffects.[1]It progressed as far as Phase 2b humanclinical trialsagainstHepatitis Cbut showed only modest benefits in comparison to existing treatments,[2][3]however it continues to be researched, and also shows activity against other viral diseases such asZika virusandfoot and mouth disease virus.[4][5]
Merimepodib was investigated in combination with remdesivir in a phase 2 clinical trial in the U.S. as a potential treatment ofCOVID-19by ViralClear Pharmaceuticals.[6][7]The trial stopped in October 2020, and the company announced in a news release that it was "unlikely that the trial would meet its primary safety endpoints", and that it "does not intend to further develop merimepodib".[8]
References
[edit]- ^Jain J, Almquist SJ, Shlyakhter D, Harding MW (May 2001). "VX-497: a novel, selective IMPDH inhibitor and immunosuppressive agent".Journal of Pharmaceutical Sciences.90(5): 625–637.doi:10.1002/1520-6017(200105)90:5<625::aid-jps1019>3.0.co;2-1.PMID11288107.
- ^McHutchison JG, Shiffman ML, Cheung RC, Gordon SC, Wright TL, Pottage JC, et al. (2005)."A randomized, double-blind, placebo-controlled dose-escalation trial of merimepodib (VX-497) and interferon- Alpha in previously untreated patients with chronic hepatitis C".Antiviral Therapy.10(5): 635–643.doi:10.1177/135965350501000503.PMID16152757.S2CID237218077.
- ^Rustgi VK, Lee WM, Lawitz E, Gordon SC, Afdhal N, Poordad F, et al. (December 2009)."Merimepodib, pegylated interferon, and ribavirin in genotype 1 chronic hepatitis C pegylated interferon and ribavirin nonresponders".Hepatology.50(6): 1719–1726.doi:10.1002/hep.23204.PMID19852040.S2CID2497811.
- ^Tong X, Smith J, Bukreyeva N, Koma T, Manning JT, Kalkeri R, et al. (January 2018). "Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens".Antiviral Research.149:34–40.doi:10.1016/j.antiviral.2017.11.004.PMID29126899.S2CID46843542.
- ^Li SF, Gong MJ, Shao JJ, Sun YF, Zhang YG, Chang HY (October 2019). "Antiviral activity of merimepodib against foot and mouth disease virus in vitro and in vivo".Molecular Immunology.114:226–232.doi:10.1016/j.molimm.2019.07.021.hdl:2268/248312.PMID31386979.S2CID199469162.
- ^Clinical trial numberNCT04410354for "Study of Merimepodib in Combination With Remdesivir in Adult Patients With Advanced COVID-19" atClinicalTrials.gov
- ^Arunachalam R, Kumar KP (10 July 2020)."COVID-19: Clinical Trials and Potential Therapeutic Agents - A Narrative Review".SSRN from Elsevier.SSRN3653031.Retrieved14 August2020.
- ^"ViralClear halts its Phase 2 Hospitalized COVID-19 Trial".BioSig Technologies, Inc. (BSGM).26 October 2020.
