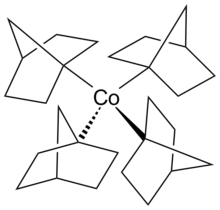
Metal tetranorbornyl
Inorganometallic chemistry,metal tetranorbornylsare compounds with the formula M(nor)4(M = a metal in a +4 oxidation state) (1-nor = 4bicyclo[2.2.1]hept-1-yl) and are one of the largest series of tetraalkyl complexes derived from identicalligands.[1][2]Metal tetranorbornyls display uniformstoichiometry,low-spinconfigurations, and highstability,which can be attributed to their +4oxidation statemetal center. The stability of metal tetranorbornyls is predominately considered to be derived from the unfavorableβ-hydride elimination.Computational calculations have determined thatLondon dispersioneffects significantly contribute to the stability of metal tetranorbornyls. Specifically, Fe(nor)4has a stabilization of 45.9 kcal/mol−1.Notable metal tetranorbornyls are those synthesized with metal centers ofcobalt,manganese,oriron.[3]
Preparation
[edit]Traditionally, metal tetranorbornyls are prepared by a reaction ofalkyllithiums,such as 1-norbornyllithium, withtransition-metalhalideswhile tumbling with glass beads inpentane.This is followed by afiltrationstep using a column of alumina to remove pentane byproducts. Lastly, arecrystallizationstep from pentane to obtain thecrystallinecompound.[1]
Alternative methods for the preparation of metal tetranorbornyls have been proposed. Specifically, the tetrakis(1-norbornyl)chromium complex can be prepared in inert atmosphere conditions with 1-norbornyllithium dissolved inhexane.An addition of CrCl3(THF)3is made and allowed to stir for 48 hours. After, the solution iscentrifugedfor the removal ofLiCl.The resultingsupernatantis applied to an alumina column with hexane being used as theelutionsolvent. The use of the alumina column allows for the collection of a purple fraction that undergoes solvent evaporation andsublimationto obtain the desired Cr(nor)4complex.[4]
Thetetrakis(1-norbornyl)cobalt(IV)complex can be prepared by the following:
- CoCl2·THF + 4norLi → [pentane] [Co(nor)4] + CO + 4 LiCl + 2THF[5]
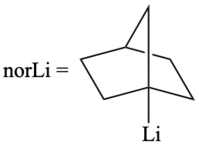
1-norbornyllithium
The tetrakis(1-norbornyl)molybdenum(IV) complex was prepared by William M. Davis,Richard R. Schrock,and Richard M. Kolodziej by the following:
- MoCl3(THF)3+ 4norLi → [Ether/ THF (30/1)] Mo(nor)4[6]
The MoCl3(THF)3was stirred with 1-norbornyllithium in a mixture ofTHFanddiethyl etherat.The reaction mixture was then warmed toand after approximately 90 minutes it was observed as a red color with a blueprecipitate.The reaction mixture was thenfilteredto remove the blue precipitate. The red filtrate was thenreducedvia a vacuum to yield red crystals of Mo(nor)4.[6]
Structure and bonding
[edit]The stability of metal tetranorbornyls is generally considered to be a result of unfavorable β-hydrogen elimination. Metal alkyl species with β-hydrogen atoms present on the alkyl group are disfavored due to β-hydrogen migration to the metal center, which results in anolefinbeing eliminated and the production of the correspondingmetal hydride.1-norbornyl does not undergo β-hydrogen migration even though it possesses 6 β-hydrogen atoms due to the unfavorable formation of the olefin, 1-norbornene. According toBredt's rule,one of the sp2carbons of the double-bonded carbon atoms would be located at the bridgehead, which would cause 1-norbornene to be highly strained.[7]β-hydrogen elimination does not explain the formation of metal tetranorbornyls complexes that are synthesized from lower valent metal centerprecursors,shortenedbond lengthsbetween the metal center and 1-norbornylligandcarbons, or the resulting low-spintetrahedral molecular geometry.[1][3]

Unfavorable β-hydrogen migration resulting in the formation of 1-norbornene from a 1-norbornyl metal complex[7]
Quantum mechanical calculationshave elucidated that London dispersion forces between the norbornyl ligands are accountable for the stability and molecular geometry of thehomoleptictetranorbornyl metal complexes.[3][7]
Metal tetranorbornyls complexes consisting of thedivalentandtrivalentmetal center species ofCr,Mn,FeandCohalidesundergo formation ofnegatively charged complexesfollowed byoxidationthat is induced by othertransition-metalspecies in the reaction. Factors that lead to disproportionation are traditionally considered to be derived from thetertiarycarbanionligand, 1-norbornyllithium, and the lack of potential for the pentane solvent to act as a ligand. Therefore, metal tetranorbornyls composed of first-row transition metals are not accessible to be penetrated by small reagents due to the metal center's coordination sphere.[1]
Tetrakis(1-norbornyl)cobalt(IV)
[edit]Tetrakis(1-norbornyl)cobalt(IV)is a thermally stablehomolepticcomplex observed withσ-bondingligands. The metal tetranorbornyl complex was the first isolated low-spin complex with tetrahedral molecular geometry. The tetrakis(1-norbornyl)cobalt(IV) complex was first synthesized by Barton K. Bower and Howard G. Tennent in 1972.[1][8][9]
Thetetrakis(1-norbornyl)cobalt(IV)oxidation state is a reversible reaction using O2as theoxidizing agent.[10]The coordination environment of the cobalt metal center has a distorted tetrahedron structure. When examined byx-ray crystallography,the metal tetranorbornyl has a crystallographicCssymmetry due to the presence of six carbons laid on the mirror plane. However, the four carbons atoms bonded to the cobalt metal center resembled a tetragonally compressed tetrahedron, which appeared as a pseudoD2dsymmetry.
The cobalt metal center in the +4 oxidation state has ad5configuration.[11]Typically, the d5configuration is expected to result in the high spin complex containing 5unpaired electronsand only 1 unpaired electron in thelow spintetrahedral complex. The single unpaired electron resides in the antibonding t2orbital, which would cause the structure to experience aJahn-Teller distortion.However, Theopold and co-workers speculated that the slight tetragonal compression could have been a result of steric interactions between norbornyl ligands and crystal packing forces.[10]
Tetrakis(1-norbornyl)iron(IV)
[edit]The tetrakis(1-norbornyl)iron(IV) complex was first synthesized by Barton K. Bower and Howard G. Tennent in 1972.[1]The 1-norbornyl ligands on the complex have a strong dispersion attraction andhigh ring strain,which as a consequence hinders the α- and β-hydride elimination reactions. Additionally, the identical ligands cause a reduced chemical reactivity due to a crowded chemical environment that impedes the interaction of small molecules with the Fe-C bonds.[12]
Synthesized complexes
[edit]Barton K. Bower and Howard G. Tennent were able to successfully synthesize and characterize the following metal tetranorbornyls derived from thefirst-,second-,andthird-row transition metals:[1]
- tetrakis(1-norbornyl)hafnium
- tetrakis(1-norbornyl)zirconium
- tetrakis(1-norbornyl)titanium
- tetrakis(1-norbornyl)vanadium
- tetrakis(1-norbornyl)chromium
- tetrakis(1-norbornyl)manganese
- tetrakis(1-norbornyl)iron
- tetrakis(1-norbornyl)molybdenum
The metal tetranorbornyls complexes ofhafnium,zirconium,titanium,andvanadiumdisplay atetrahedral molecular geometry,which is analogous to thetetrachlorideform of the metals. In comparison, thecobalt,manganese,andironcomplexes display a tetragonal molecular geometry.[1]A combination of London dispersion force and steric effects from the 1-norbornyl ligands results in the stability observed for the metal center.[3]
Characterization
[edit]Magnetic measurements
[edit]The resultingmolecular geometryof the metal tetranorbornyls complexes is due to theunpairedandpairedd electrons.Magnetic measurementshave indicated that the d electrons of tetrakis(1-norbornyl)chromium (d2) and tetrakis(1-norbornyl)manganese (d3) are not spin paired. The four d electrons of tetrakis(1-norbornyl)iron and tetrakis(1-norbornyl)cobalt are spin paired.[1]
Electron paramagnetic resonance spectroscopy
[edit]Metal tetranorbornyls are commonly characterized viaelectron paramagnetic resonance(EPR) spectroscopy. Tetrakis(1-norbornyl)molybdenum was observed as a room temperature EPR signal that originated from a d2metal center, which was considered to have two unpaired electrons in the egorbital. In addition, the resulting EPR signal of tetrakis(1-norbornyl)chromium was comparable.[6][13]
Cyclic voltammetry
[edit]In 1988, Klaus H. Theopold and Erin K. Byrne performed the electrochemical experiment,cyclic voltammetry,to determine how oxidizing was the metal center of the tetrakis(1-norbornyl)cobalt(IV) complex. Two reversibleelectron transferwaves at -0.65 and -2.02 V were observed in THF, which elucidated that the difference in peak potentials were consistent with two one-electron transfer processes when being compared to the ferricenium/ferrocenecouple.[5]In the same year, William M. Davis, Richard R. Schrock, and Richard M. Kolodziej produced acyclic voltammogramfor tetrakis(1-norbornyl)molybdenum. Two oxidation waves were observed at -0.15 and +1.25 V inDCM.The oxidation at -0.15 V was considered to be reversible. In comparison, the second oxidation at +1.25 V was considered to be irreversible.[6]
References
[edit]- ^abcdefghiBower, Barton K.; Tennent, Howard G. (April 1972)."Transition metal bicyclo[2.2.1]hept-1-yls".Journal of the American Chemical Society.94(7): 2512–2514.doi:10.1021/ja00762a056.ISSN0002-7863.
- ^Abrahamson, Harmon B.; Brandenburg, Kathryn L.; Lucero, Barbara; Martin, Mary E.; Dennis, Eleonore (September 1984)."Spectroscopy and photochemistry of the tetranorbornyl complexes of titanium and chromium".Organometallics.3(9): 1379–1386.doi:10.1021/om00087a010.ISSN0276-7333.
- ^abcdLiptrot, David J.; Guo, Jing‐Dong; Nagase, Shigeru; Power, Philip P. (2016-11-14)."Dispersion Forces, Disproportionation, and Stable High‐Valent Late Transition Metal Alkyls".Angewandte Chemie International Edition.55(47): 14766–14769.doi:10.1002/anie.201607360.ISSN1433-7851.PMID27778428.
- ^Brandenburg, Kathryn Lynn.Photoinduced reactions of group VI organotransition metal complexes.OCLC83966357.
- ^abByrne, Erin K.; Theopold, Klaus H. (February 1987)."Redox chemistry of tetrakis(1-norbornyl)cobalt. Synthesis and characterization of a cobalt(V) alkyl and self-exchange rate of a Co(III)/Co(IV) couple".Journal of the American Chemical Society.109(4): 1282–1283.doi:10.1021/ja00238a066.ISSN0002-7863.
- ^abcdKolodziej, R. M.; Schrock, R. R.; Davis, W. M. (1988-12-27)."ChemInform Abstract: Synthesis and Characterization of Mo(nor)4 (nor: 1-Norbornyl)".ChemInform.19(52).doi:10.1002/chin.198852222.ISSN0931-7597.
- ^abc"Dispersion Effects in Stabilizing Organometallic Compounds: Tetra-1-norbornyl Derivatives of the First-Row Transition Metals as Exceptional Examples".doi:10.1021/acs.jpca.9b06769.s001.Retrieved2023-03-12.
{{cite journal}}:Cite journal requires|journal=(help) - ^Byrne, Erin K.; Richeson, Darrin S.; Theopold, Klaus H. (1986)."Tetrakis(1-norbornyl)cobalt, a low spin tetrahedral complex of a first row transition metal".Journal of the Chemical Society, Chemical Communications(19): 1491–1492.doi:10.1039/c39860001491.ISSN0022-4936.
- ^Byrne, Erin K.; Theopold, Klaus H. (May 1989)."Synthesis, characterization, and electron-transfer reactivity of norbornyl complexes of cobalt in unusually high oxidation states".Journal of the American Chemical Society.111(11): 3887–3896.doi:10.1021/ja00193a021.ISSN0002-7863.
- ^abByrne, Erin K.; Richeson, Darrin S.; Theopold, Klaus H. (1986)."Tetrakis(1-norbornyl)cobalt, a low spin tetrahedral complex of a first row transition metal".Journal of the Chemical Society, Chemical Communications(19): 1491.doi:10.1039/c39860001491.ISSN0022-4936.
- ^Green, Malcolm L. H.; Parkin, Gerard (2014-04-28)."Application of the Covalent Bond Classification Method for the Teaching of Inorganic Chemistry".Journal of Chemical Education.91(6): 807–816.Bibcode:2014JChEd..91..807G.doi:10.1021/ed400504f.ISSN0021-9584.
- ^Li, Huidong; Wang, Linshen; Hu, Yucheng; Zhang, Ze; Wan, Di; Fan, Qunchao; King, R. Bruce; Schaefer, Henry F. (2020-08-27)."Comparative Study of the Thermal Stabilities of the Experimentally Known High-Valent Fe(IV) Compounds Fe(1-norbornyl) 4 and Fe(cyclohexyl) 4".The Journal of Physical Chemistry A.124(34): 6867–6876.Bibcode:2020JPCA..124.6867L.doi:10.1021/acs.jpca.0c04055.ISSN1089-5639.PMID32786998.S2CID221127617.
- ^Ward, G. A.; Bower, B. K.; Findlay, M.; Chien, James C. W. (1974-05-07)."ChemInform Abstract: Electron Paramagnetic Resonance of Tetrakis(1-norbornyl)Chromium".Chemischer Informationsdienst.5(18).doi:10.1002/chin.197418348.ISSN0009-2975.