Metal


| Part ofa serieson the |
| Periodic table |
|---|
Ametal(fromAncient Greekμέταλλον(métallon)'mine, quarry, metal') is amaterialthat, when polished or fractured, shows a lustrous appearance, and conductselectricityandheatrelatively well. These properties are all associated with having electrons available at theFermi level,as against nonmetallic materials which do not.[1]: Chpt 8 & 19 [2]: Chpt 7 & 8 Metals are typicallyductile(can be drawn into wires) andmalleable(they can be hammered into thin sheets).[3]
A metal may be achemical elementsuch asiron;analloysuch asstainless steel;or a molecular compound such aspolymeric sulfur nitride.[4]The general science of metals is calledmetallurgy,a subtopic ofmaterials science;aspects of the electronic and thermal properties are also within the scope ofcondensed matter physicsandsolid-state chemistry,it is amultidisciplinarytopic. In colloquial use materials such as steel alloys are referred to as metals, while others such as polymers, wood or ceramics arenonmetallic materials.
A metal conducts electricity at a temperature ofabsolute zero,[5]which is a consequence of the states at the Fermi energy.[1][2]Many elements and compounds become metallic under high pressures, for example,iodinegradually becomes a metal at a pressure of between 40 and 170 thousand timesatmospheric pressure.Sodiumbecomes a nonmetal at pressure of just under two million times atmospheric pressure, and at even higher pressures it is expected to become a metal again.
When discussing theperiodic tableand some chemical properties the term metal is often used to denote those elements which in pure form and at standard conditions are metals in the sense of electrical conduction mentioned above. The related term metallic may also be used for types ofdopantatoms or alloying elements.
Inastronomymetal refers to all chemical elements in a star that are heavier thanhelium.In this sense the first four "metals" collecting in stellar cores through nucleosynthesis arecarbon,nitrogen,oxygen,andneon.A starfuseslighter atoms, mostly hydrogen and helium, into heavier atoms over its lifetime. Themetallicityof an astronomical object is the proportion of its matter made up of the heavier chemical elements.[6][7]
The strength and resilience of some metals has led to their frequent use in, for example, high-rise building and bridgeconstruction,as well as most vehicles, manyhome appliances,tools, pipes, and railroad tracks.Precious metalswere historically used ascoinage,but in the modern era,coinage metalshave extended to at least 23 of the chemical elements.[8]There is also extensive use of multi-element metals such astitanium nitride[9]ordegenerate semiconductorsin the semiconductor industry.
The history of refined metals is thought to begin with the use of copper about 11,000 years ago. Gold, silver, iron (as meteoric iron), lead, and brass were likewise in use before the first known appearance of bronze in the fifth millennium BCE. Subsequent developments include the production of early forms of steel; the discovery ofsodium—the firstlight metal—in 1809; the rise of modernalloy steels;and, since the end of World War II, the development of more sophisticated alloys.
Properties
Form and structure

Most metals are shiny andlustrous,at least when polished, or fractured. Sheets of metal thicker than a fewmicrometresappear opaque, butgold leaftransmits green light. This is due to the freely moving electrons which reflect light.[1][2]
Although most elemental metals have higherdensitiesthannonmetals,[10]there is a wide variation in their densities,lithiumbeing the least dense (0.534 g/cm3) andosmium(22.59 g/cm3) the most dense. Some of the6d transition metalsare expected to be denser than osmium, but their known isotopes are too unstable for bulk production to be possible[11]Magnesium, aluminium and titanium arelight metalsof significant commercial importance. Their respective densities of 1.7, 2.7, and 4.5 g/cm3can be compared to those of the older structural metals, like iron at 7.9 and copper at 8.9 g/cm3.An iron ball would thus weigh about as much as three aluminum balls of equal volume.

(a) Brittle fracture
(b) Ductile fracture
(c) Completely ductile fracture
Metals are typically malleable and ductile, deforming under stress withoutcleaving.[10]The nondirectional nature of metallic bonding contributes to the ductility of most metallic solids, where thePeierls stressis relatively low allowing fordislocationmotion, and there are also many combinations of planes and directions forplastic deformation.[12]Due to their having close packed arrangements of atoms theBurgers vectorof the dislocations are fairly small, which also means that the energy needed to produce one is small.[3][12]In contrast, in an ionic compound like table salt the Burgers vectors are much larger and the energy to move a dislocation is far higher.[3]Reversibleelastic deformationin metals can be described well byHooke's Lawfor the restoring forces, where thestressis linearly proportional to thestrain.[13]
A temperature change may lead to the movement ofstructural defectsin the metal such asgrain boundaries,point vacancies,line and screw dislocations,stacking faultsandtwinsin bothcrystallineandnon-crystallinemetals. Internalslip,creep,andmetal fatiguemay also ensue.[3][12]
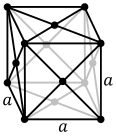
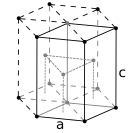
The atoms of simple metallic substances are often in one of three commoncrystal structures,namelybody-centered cubic(bcc),face-centered cubic(fcc), andhexagonal close-packed(hcp). In bcc, each atom is positioned at the center of a cube of eight others. In fcc and hcp, each atom is surrounded by twelve others, but the stacking of the layers differs. Some metals adopt different structures depending on the temperature.[14]
-
Body-centered cubic crystal structure, with a 2-atom unit cell, as found in e.g. chromium, iron, and tungsten
-
Face-centered cubic crystal structure, with a 4-atom unit cell, as found in e.g. aluminum, copper, and gold
-
Hexagonal close-packed crystal structure, with a 6-atom unit cell, as found in e.g. titanium, cobalt, and zinc
Many other metals with different elements have more complicated structures, such asrock-salt structureintitanium nitrideorperovskite (structure)in some nickelates.[15]
Electrical and thermal

The electronic structure of metals means they are relatively goodconductors of electricity.The electrons all have differentmomenta,which average to zero when there is no externalvoltage.When a voltage is applied some move a little faster in a given direction, some a little slower so there is a netdrift velocitywhich leads to an electric current.[1][2]This involves small changes in whichwavefunctionsthe electrons are in, changing to those with the higher momenta.Quantum mechanicsdictates that one can only have one electron in a given state, thePauli exclusion principle.[16]Therefore there have to be emptydelocalized electronstates (with the higher momenta) available at the highest occupied energies as sketched in the Figure. In a semiconductor like silicon or a nonmetal likestrontium titanatethere is an energy gap between the highest filled states of the electrons and the lowest unfilled, so no accessible states with slightly higher momenta. Consequently, semiconductors and nonmetals are poor conductors, although they can carry some current when doped with elements that introduce additional partially occupied energy states at higher temperatures.[17]There are also materials such as graphite where there are available states with higher moments for movement in the graphitic planes, but none for normal to them.[18]
The elemental metals have electrical conductivity values of from 6.9 × 103S/cm formanganeseto 6.3 × 105S/cm forsilver.In contrast, asemiconductingmetalloid such asboronhas an electrical conductivity 1.5 × 10−6S/cm. With one exception, metallic elements reduce their electrical conductivity when heated.Plutoniumincreases its electrical conductivity when heated in the temperature range of around −175 to +125 °C, with anomalously large thermal expansion coefficient and a phase change from monoclinic to face-centered cubic near 100 °C.[19]

All of the metallic alloys as well as conducting ceramics and polymers are metals by the same definition; for instancetitanium nitridehas delocalized states at the Fermi level. They have electrical conductivities similar to those of elemental metals. Liquid forms are also metallic conductors or electricity, for instancemercury.In normal conditions no gases are metallic conductors. However, aplasma (physics)is a metallic conductor and the charged particles in a plasma have many properties in common with those of electrons in elemental metals, particularly for white dwarf stars.[20]
Metals are relatively goodconductors of heat,which in metals is transported mainly by the conduction electrons.[21]At higher temperatures the electrons can occupy slightly higher energy levels given byFermi–Dirac statistics.[2][17]These have slightly higher momenta (kinetic energy) and can pass on thermal energy. The empiricalWiedemann–Franz lawstates that in many metals the ratio between thermal and electrical conductivities is proportional to temperature, with a proportionality constant that is roughly the same for all metals.[2]
The contribution of a metal's electrons to its heat capacity and thermal conductivity, and the electrical conductivity of the metal itself can be approximately calculated from thefree electron model.[2]However, this does not take into account the detailed structure of the metal's ion lattice. Taking into account the positive potential caused by the arrangement of the ion cores enables consideration of theelectronic band structureandbinding energyof a metal. Various models are applicable, the simplest being thenearly free electron model.[2]Modern methods such asdensity functional theoryare typically used.
Chemical
The elements which form metals usually formcationsthrough electron loss.[10]Most will react with oxygen in the air to formoxidesover various timescales (potassiumburns in seconds while ironrustsover years) which depend upon whether the native oxide forms apassivation layerthat acts as adiffusion barrier.[22][23]Some others, likepalladium,platinum,andgold,do not react with the atmosphere at all; gold can form compounds where it gains an electron (aurides, e.g.caesium auride). Theoxidesof elemental metals are oftenbasic,excepting oxides with very highoxidation statessuch as CrO3,Mn2O7,and OsO4,which have strictly acidic reactions; and oxides of the less electropositive metals such as BeO, Al2O3,and PbO, which can display both basic and acidic properties. These are termedamphotericoxides.
Periodic table distribution
The elements that form exclusively metallic structures under ordinary conditions are shown in yellow on the periodic table below. The remaining elements either formcovalent networkstructures (light blue), molecular covalent structures (dark blue), or remain as single atoms (violet).[24]Astatine (At), francium (Fr), and the elements from fermium (Fm) onwards are shown in gray because they are extremely radioactive and have never been produced in bulk. Theoretical and experimental evidence suggests that almost all these uninvestigated elements should be metals,[25]though there is some doubt for oganesson (Og).[26]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group→ | ||||||||||||||||||||||||||||||||
| ↓Period | ||||||||||||||||||||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
Metallic Network covalent Molecularcovalent Single atoms Unknown Background colorshows bonding of simple substances in theperiodic table.If there are several, the most stable allotrope is considered.
The situation changes with pressure: at extremely high pressures, all elements (and indeed all substances) are expected to metallize.[25]Arsenic (As) has both a stable metallic allotrope and a metastable semiconducting allotrope at standard conditions. A similar situation affects carbon (C):graphiteis metallic, butdiamondis not.
Alloys

In the context of metals, an alloy is a substance having metallic properties which is composed of two or moreelements.Often at least one of these is a metallic element; the term "alloy" is sometimes used more generally as insilicon–germaniumalloys. An alloy may have a variable or fixed composition. For example, gold and silver form an alloy in which the proportions of gold or silver can be varried; titanium and silicon form an alloyTiSi2in which the ratio of the two components is fixed (also known as anintermetallic compound).

Most pure metals are either too soft, brittle, or chemically reactive for practical use. Combining different ratios of metals as alloys modifies the properties of pure metals to produce desirable characteristics. The aim of making alloys is generally to make them less brittle, harder, resistant to corrosion, or have a more desirable color and luster. Of all the metallic alloys in use today, the alloys ofiron(steel,stainless steel,cast iron,tool steel,alloy steel) make up the largest proportion both by quantity and commercial value. Iron alloyed with various proportions of carbon gives low-, mid-, and high-carbon steels, with increasing carbon levels reducing ductility and toughness. The addition ofsiliconwill produce cast irons, while the addition ofchromium,nickel,andmolybdenumto carbon steels (more than 10%) results in stainless steels.
Other significant metallic alloys are those ofaluminum,titanium,copper,andmagnesium.Copper alloys have been known since prehistory—bronzegave theBronze Ageits name—and have many applications today, most importantly in electrical wiring. The alloys of the other three metals have been developed relatively recently; due to their chemical reactivity they needelectrolyticextraction processes. The alloys of aluminum, titanium, and magnesium are valued for their high strength-to-weight ratios; magnesium can also provideelectromagnetic shielding.[27][28]These materials are ideal for situations where high strength-to-weight ratio is more important than material cost, such as in aerospace and some automotive applications.
Alloys specially designed for highly demanding applications, such asjet engines,may contain more than ten elements.
Categories
Metals can be categorised according to their composition, physical or chemical properties. Categories described in the subsections below includeferrousandnon-ferrousmetals; brittle metals andrefractory metals;white metals;heavyandlightmetals;base,noble,andpreciousmetals as well as both metallicceramicsandpolymers.
Ferrous and non-ferrous metals
The term "ferrous" is derived from theLatinword meaning "containing iron". This can include pure iron, such aswrought iron,or an alloy such assteel.Ferrous metals are oftenmagnetic,but not exclusively. Non-ferrous metals and alloys lack appreciable amounts of iron.
Brittle elemental metal
While nearly all elemental metals are malleable or ductile, a few—beryllium, chromium, manganese, gallium, and bismuth—are brittle.[29]Arsenic and antimony, if admitted as metals, are brittle. Low values of the ratio of bulkelastic modulustoshear modulus(Pugh's criterion) are indicative of intrinsic brittleness. A material is brittle if it is hard for dislocations to move, which is often associated with largeBurgers vectorsand only a limited number of slip planes.[30]
Refractory metal
A refractory metal is a metal that is very resistant to heat and wear. Which metals belong to this category varies; the most common definition includes niobium, molybdenum, tantalum, tungsten, and rhenium as well as their alloys. They all have melting points above 2000 °C, and a highhardnessat room temperature. Several compounds such as titanium nitride are also described as refractory metals.
-
Niobium crystals and a 1 cm3anodizedniobium cube for comparison
-
Molybdenum crystals and a 1 cm3molybdenum cube for comparison
-
Tantalum single crystal, some crystalline fragments, and a 1 cm3tantalum cube for comparison
-
Tungsten rods with evaporated crystals, partially oxidized with colorful tarnish, and a 1 cm3tungsten cube for comparison
-
Rhenium single crystal, a remelted bar, and a 1 cm3rhenium cube for comparison
-
Titanium nitride powder
White metal
Awhite metalis any of a range of white-colored alloys with relatively low melting points used mainly for decorative purposes.[31][32]In Britain, the fine art trade uses the term "white metal" in auction catalogues to describe foreign silver items which do not carry British Assay Office marks,[33]but which are nonetheless understood to be silver and are priced accordingly.
Heavy and light metals
A heavy metal is any relatively dense metal ormetalloid.[34]More specific definitions have been proposed, but none have obtained widespread acceptance. Some heavy metals have niche uses, or are notably toxic; some are essential in trace amounts. All other metals are light metals.
Base, noble, and precious metals
The termbase metalrefers to a metal that is easilyoxidizedorcorroded,such as reacting easily with dilutehydrochloric acid(HCl) to form a metal chloride andhydrogen.Examples include iron,nickel,lead,and zinc. Copper is considered a base metal as it is oxidized relatively easily, although it does not react with HCl.

The termnoble metalis commonly used in opposition tobase metal.Noble metals are less reactive, resistant tocorrosionoroxidation,[35]unlike mostbase metals.They tend to be precious metals, often due to perceived rarity. Examples include gold, platinum, silver,rhodium,iridium, and palladium.
Inalchemyandnumismatics,the term base metal is contrasted withprecious metal,that is, those of high economic value.[36]Most coins today are made of base metals withlow intrinsic value;in the past, coins frequently derived their value primarily from theirprecious metalcontent;gold,silver,platinum,andpalladiumeach have anISO 4217currency code. Currently they have industrial uses such as platinum and palladium incatalytic converters,are used injewelleryand also a role as investments and astore of value.[37]Palladium and platinum, as of summer 2024, were valued at slightly less than half quarters the price of gold, while silver is substantially less expensive.
Valve metals
In electrochemistry, a valve metal is a metal which passes current in only one direction.
Ceramic metals

There are many compounds which have metallic electrical conduction, but are not simple combinations of metallic elements. (They are not the same ascermetswhich are composites of a non-conducting ceramic and a conducting metal.) One set such as many of the transition metal nitrides has significant ionic character to the bonding, so can be classified as both ceramics and metals.[9]They have partially filled states at the Fermi level[9]so are good thermal and electrical conductors, and often significant charge transfer from the transition metal atoms to the nitrogen.[9]However, unlike most elemental metals, ceramic metals are often not particularly ductile. Their uses are widespread, for instancetitanium nitridefinds use in orthopedic devices[38]and as a wear resistant coating.[39]In many cases their utility depends upon there being effective deposition methods so they can be used as thin film coatings.[40]
Polymer metals

There are many polymers which have metallic electrical conduction,[42][43]typically associated with extended aromatic components such as in the polymers indicated in the Figure. The conduction of the aromatic regions is similar to that of graphite, so is highly directional.[44]
Half metal
Ahalf-metalis any substance that acts as aconductortoelectronsof onespinorientation, but as aninsulatororsemiconductorto those of the opposite spin. They were first described in 1983, as an explanation for the electrical properties ofmanganese-basedHeusler alloys.[45]Although all half-metals areferromagnetic(orferrimagnetic), most ferromagnets are not half-metals. Many of the known examples of half-metals areoxides,sulfides,orHeusler alloys.[46]
Semimetal
Asemimetalis a material with a small energy overlap between the bottom of theconductionbandand the top of thevalence band,but they do not overlap inmomentum space.Unlike a regularmetal,semimetals have charge carriers of both types (holes and electrons), so that one could also argue that they should be called 'double-metals' rather than semimetals. However, the charge carriers typically occur in much smaller numbers than in a real metal. In this respect they resembledegenerate semiconductorsmore closely. This explains why the electrical properties of semimetals are partway between those of metals andsemiconductors.There are additional types, in particularWeylandDirac semimetals.[47]
The classic semimetallic elements arearsenic,antimony,bismuth,α-tin(gray tin) andgraphite.There are alsochemical compounds,such asmercury telluride(HgTe),[48]and someconductive polymerscan behave as semimetals.[49]
Lifecycle
Formation
| abundance and main occurrence or source, by weight[n 1] | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
| 1 | H | He | |||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||
| 6 | Cs | Ba | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | ||||
| 7 | |||||||||||||||||||
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | |||||||
| Th | U | ||||||||||||||||||
Most abundant (up to82000ppm)
| |||||||||||||||||||
Abundant (100–999ppm)
| |||||||||||||||||||
Uncommon (1–99 ppm)
| |||||||||||||||||||
Rare (0.01–0.99ppm)
| |||||||||||||||||||
Very rare (0.0001–0.0099ppm)
| |||||||||||||||||||
| Metals left of the dividing line occur (or are sourced) mainly aslithophiles;those to the right, aschalcophilesexcept gold (asiderophile) and tin (a lithophile). | |||||||||||||||||||
Metallic elements up to thevicinity of iron(in the periodic table) are largely made viastellar nucleosynthesis.In this process, lighter elements from hydrogen tosiliconundergo successivefusionreactions inside stars, releasing light and heat and forming heavier elements with higher atomic numbers.[50]
Heavier elements are not usually formed this way since fusion reactions involving such nuclei would consume rather than release energy.[51]Rather, they are largely synthesised (from elements with a lower atomic number) byneutron capture,with the two main modes of this repetitive capture being thes-processand ther-process.In the s-process ( "s" stands for "slow" ), singular captures are separated by years or decades, allowing the less stable nuclei tobeta decay,[52]while in the r-process ( "rapid" ), captures happen faster than nuclei can decay. Therefore, the s-process takes a more-or-less clear path: for example, stable cadmium-110 nuclei are successively bombarded by free neutrons inside a star until they form cadmium-115 nuclei which are unstable and decay to form indium-115 (which is nearly stable, with a half-life30000times the age of the universe). These nuclei capture neutrons and form indium-116, which is unstable, and decays to form tin-116, and so on.[50][53][n 2]In contrast, there is no such path in the r-process. The s-process stops at bismuth due to the short half-lives of the next two elements, polonium and astatine, which decay to bismuth or lead. The r-process is so fast it can skip this zone of instability and go on to create heavier elements such asthoriumand uranium.[55]
Metals condense in planets as a result of stellar evolution and destruction processes. Stars lose much of their mass when it isejectedlate in their lifetimes, and sometimes thereafter as a result of aneutron starmerger,[56][n 3]thereby increasing the abundance of elements heavier than helium in theinterstellar medium.When gravitational attraction causes this matter to coalesce and collapsenew stars and planets are formed.[58]
Abundance and occurrence

TheEarth's crustis made of approximately 25% of metallic elements by weight, of which 80% are light metals such as sodium, magnesium, and aluminium. Despite the overall scarcity of some heavier metals such as copper, they can become concentrated in economically extractable quantities as a result of mountain building, erosion, or other geological processes.
Metallic elements are primarily found as lithophiles (rock-loving) or chalcophiles (ore-loving). Lithophile elements are mainly the s-block elements, the more reactive of the d-block elements, and the f-block elements. They have a strong affinity for oxygen and mostly exist as relatively low-density silicate minerals. Chalcophile elements are mainly the less reactive d-block elements, and the period 4–6 p-block metals. They are usually found in (insoluble) sulfide minerals. Being denser than the lithophiles, hence sinking lower into the crust at the time of its solidification, the chalcophiles tend to be less abundant than the lithophiles.
On the other hand, gold is a siderophile, or iron-loving element. It does not readily form compounds with either oxygen or sulfur. At the time of the Earth's formation, and as the most noble (inert) of metallic elements, gold sank into the core due to its tendency to form high-density metallic alloys. Consequently, it is relatively rare. Some other (less) noble ones—molybdenum, rhenium, the platinum group metals (ruthenium, rhodium, palladium, osmium, iridium, and platinum), germanium, and tin—can be counted as siderophiles but only in terms of their primary occurrence in the Earth (core, mantle, and crust), rather the crust. These otherwise occur in the crust, in small quantities, chiefly as chalcophiles (less so in their native form).[n 4]
The rotating fluid outer core of the Earth's interior, which is composed mostly of iron, is thought to be the source of Earth's protective magnetic field.[n 5]The core lies above Earth's solid inner core and below its mantle. If it could be rearranged into a column having a 5 m2(54 sq ft) footprint it would have a height of nearly 700 light years. The magnetic field shields the Earth from the charged particles of the solar wind, and cosmic rays that would otherwise strip away the upper atmosphere (including the ozone layer that limits the transmission of ultraviolet radiation).
Extraction
Metallic elements are often extracted from the Earth by means of mining ores that are rich sources of the requisite elements, such asbauxite.Ore is located byprospectingtechniques, followed by the exploration and examination of deposits. Mineral sources are generally divided intosurface mines,which are mined by excavation using heavy equipment, andsubsurface mines.In some cases, the sale price of the metal(s) involved make it economically feasible to mine lower concentration sources.
Once the ore is mined, the elements must beextracted,usually by chemical or electrolytic reduction.Pyrometallurgyuses high temperatures to convert ore into raw metals, whilehydrometallurgyemploysaqueouschemistry for the same purpose.
When a metallic ore is an ionic compound, the ore must usually besmelted—heated with a reducing agent—to extract the pure metal. Many common metals, such as iron, are smelted usingcarbonas a reducing agent. Some metals, such as aluminum andsodium,have no commercially practical reducing agent, and are extracted usingelectrolysisinstead.[59][60]
Sulfideores are not reduced directly to the metal but are roasted in air to convert them to oxides.
Recycling

Demand for metals is closely linked to economic growth given their use in infrastructure, construction, manufacturing, and consumer goods. During the 20th century, the variety of metals used in society grew rapidly. Today, the development of major nations, such as China and India, and technological advances, are fueling ever more demand. The result is that mining activities are expanding, and more and more of the world's metal stocks are above ground in use, rather than below ground as unused reserves. An example is the in-use stock ofcopper.Between 1932 and 1999, copper in use in the U.S. rose from 73 g to 238 g per person.[61]
Metals are inherently recyclable, so in principle, can be used over and over again, minimizing these negative environmental impacts and saving energy. For example, 95% of the energy used to make aluminum from bauxite ore is saved by using recycled material.[62]
Globally, metal recycling is generally low. In 2010, theInternational Resource Panel,hosted by theUnited Nations Environment Programmepublished reports on metal stocks that exist within society[63]and their recycling rates.[61]The authors of the report observed that the metal stocks in society can serve as huge mines above ground. They warned that the recycling rates of some rare metals used in applications such as mobile phones, battery packs for hybrid cars and fuel cells are so low that unless future end-of-life recycling rates are dramatically stepped up these critical metals will become unavailable for use in modern technology.
History
Prehistory
Copper, which occurs in native form, may have been the first metal discovered given its distinctive appearance, heaviness, and malleability compared to other stones or pebbles. Gold, silver, and iron (as meteoric iron), and lead were likewise discovered in prehistory. Forms ofbrass,an alloy of copper and zinc made by concurrently smelting the ores of these metals, originate from this period (although pure zinc was not isolated until the 13th century). The malleability of the solid metals led to the first attempts to craft metal ornaments, tools, and weapons. Meteoric iron containing nickel was discovered from time to time and, in some respects this was superior to any industrial steel manufactured up to the 1880s when alloy steels become prominent.[64]
-
Gold crystals
-
Crystalline silver
-
A slice of meteoric iron
-
A brass weight (35 g)
Antiquity

The discovery ofbronze(an alloy of copper with arsenic or tin) enabled people to create metal objects which were harder and more durable than previously possible. Bronze tools, weapons, armor, andbuilding materialssuch as decorative tiles were harder and more durable than their stone and copper ( "Chalcolithic") predecessors. Initially, bronze was made of copper andarsenic(formingarsenic bronze) by smelting naturally or artificially mixed ores of copper and arsenic.[65]The earliestartifactsso far known come from theIranian plateauin the fifth millennium BCE.[66]It was only later thattinwas used, becoming the major non-copper ingredient of bronze in the late third millennium BCE.[67]Pure tin itself was first isolated in 1800 BCE by Chinese and Japanese metalworkers.
Mercury was known to ancient Chinese and Indians before 2000 BCE, and found in Egyptian tombs dating from 1500 BCE.
The earliest known production of steel, an iron-carbon alloy, is seen in pieces of ironware excavated from anarchaeological siteinAnatolia(Kaman-Kalehöyük) and are nearly 4,000 years old, dating from 1800 BCE.[68][69]
From about 500 BCE sword-makers ofToledo, Spain,were making early forms ofalloy steelby adding a mineral calledwolframite,which contained tungsten and manganese, to iron ore (and carbon). The resultingToledo steelcame to the attention of Rome when used by Hannibal in thePunic Wars.It soon became the basis for the weaponry of Roman legions; such swords were, "stronger in composition than any existing sword and, because… [they] would not break, provided a psychological advantage to the Roman soldier."[70]
In pre-Columbian America,objects made oftumbaga,an alloy of copper and gold, started being produced in Panama and Costa Rica between 300 and 500 CE. Small metal sculptures were common and an extensive range of tumbaga (and gold) ornaments comprised the usual regalia of persons of high status.
At around the same time indigenous Ecuadorians were combining gold with a naturally-occurring platinum alloy containing small amounts of palladium, rhodium, and iridium, to produce miniatures and masks composed of a white gold-platinum alloy. The metal workers involved heated gold withgrainsof the platinum alloy until the gold melted at which point the platinum group metals became bound within the gold. After cooling, the resulting conglomeration was hammered and reheated repeatedly until it became as homogenous as if all of the metals concerned had been melted together (attaining the melting points of the platinum group metals concerned was beyond the technology of the day).[71][n 7]
-
A droplet of solidified molten tin
-
-
Electrum, a natural alloy of silver and gold, was often used for making coins. Shown is the Roman god Apollo, and on the obverse, a Delphi tripod (c. 310–305 BCE).
-
A plate made ofpewter,an alloy of 85–99% tin and (usually) copper. Pewter was first used around the beginning of the Bronze Age in the Near East.
-
A pectoral (ornamental breastplate) made oftumbaga,an alloy of gold and copper
Middle Ages
Gold is for the mistress—silver for the maid—
Copper for the craftsman cunning at his trade.
"Good!" said the Baron, sitting in his hall,
"But Iron—Cold Iron—is master of them all."
Arabic and medievalalchemistsbelieved that all metals and matter were composed of the principle of sulfur, the father of all metals and carrying the combustible property, and the principle of mercury, the mother of all metals[n 8]and carrier of the liquidity, fusibility, and volatility properties. These principles were not necessarily the common substancessulfurandmercuryfound in most laboratories. This theory reinforced the belief that all metals were destined to become gold in the bowels of the earth through the proper combinations of heat, digestion, time, and elimination of contaminants, all of which could be developed and hastened through the knowledge and methods of alchemy.[n 9]
Arsenic, zinc, antimony, and bismuth became known, although these were at first called semimetals or bastard metals on account of their immalleability. All four may have been used incidentally in earlier times without recognising their nature.Albertus Magnusis believed to have been the first to isolate arsenic from a compound in 1250, by heating soap together witharsenic trisulfide.Metallic zinc, which is brittle if impure, was isolated in India by 1300 AD. The first description of a procedure for isolating antimony is in the 1540 bookDe la pirotechniabyVannoccio Biringuccio.Bismuth was described by Agricola inDe Natura Fossilium(c. 1546); it had been confused in early times with tin and lead because of its resemblance to those elements.
-
Arsenic, sealed in a container to prevent tarnishing
-
Zinc fragments and a 1 cm3cube
-
Antimony, showing its brilliant lustre
-
Bismuth in crystalline form, with a very thin oxidation layer, and a 1 cm3bismuth cube
The Renaissance




The first systematic text on the arts of mining and metallurgy wasDe la Pirotechnia(1540) byVannoccio Biringuccio,which treats the examination, fusion, and working of metals.
Sixteen years later,Georgius AgricolapublishedDe Re Metallicain 1556, a clear and complete account of the profession of mining, metallurgy, and the accessory arts and sciences, as well as qualifying as the greatest treatise on the chemical industry through the sixteenth century.
He gave the following description of a metal in hisDe Natura Fossilium(1546):
Metal is a mineral body, by nature either liquid or somewhat hard. The latter may be melted by the heat of the fire, but when it has cooled down again and lost all heat, it becomes hard again and resumes its proper form. In this respect it differs from the stone which melts in the fire, for although the latter regain its hardness, yet it loses its pristine form and properties.
Traditionally there are six different kinds of metals, namely gold, silver, copper, iron, tin, and lead. There are really others, forquicksilveris a metal, although the Alchemists disagree with us on this subject, andbismuthis also. The ancient Greek writers seem to have been ignorant of bismuth, wherefore Ammonius rightly states that there are many species of metals, animals, and plants which are unknown to us.Stibiumwhen smelted in the crucible and refined has as much right to be regarded as a proper metal as is accorded to lead by writers. If when smelted, a certain portion be added to tin, a bookseller's alloy is produced from which the type is made that is used by those who print books on paper.
Each metal has its own form which it preserves when separated from those metals which were mixed with it. Therefore neitherelectrumnor Stannum [not meaning our tin] is of itself a real metal, but rather an alloy of two metals. Electrum is an alloy of gold and silver, Stannum of lead and silver. And yet if silver be parted from the electrum, then gold remains and not electrum; if silver be taken away from Stannum, then lead remains and not Stannum.
Whether brass, however, is found as a native metal or not, cannot be ascertained with any surety. We only know of the artificial brass, which consists of copper tinted with the colour of the mineralcalamine.And yet if any should be dug up, it would be a proper metal. Black and white copper seem to be different from the red kind.
Metal, therefore, is by nature either solid, as I have stated, or fluid, as in the unique case of quicksilver.
But enough now concerning the simple kinds.[73]
Platinum, the third precious metal after gold and silver, was discovered in Ecuador during the period 1736 to 1744, by the Spanish astronomer Antonio de Ulloa and his colleague the mathematician Jorge Juan y Santacilia. Ulloa was the first person to write a scientific description of the metal, in 1748.
In 1789, the German chemist Martin Heinrich Klaproth isolated an oxide of uranium, which he thought was the metal itself. Klaproth was subsequently credited as the discoverer of uranium. It was not until 1841, that the French chemist Eugène-Melchior Péligot, prepared the first sample of uranium metal. Henri Becquerel subsequently discovered radioactivity in 1896 by using uranium.
In the 1790s, Joseph Priestley and the Dutch chemist Martinus van Marum observed the transformative action of metal surfaces on the dehydrogenation of alcohol, a development which subsequently led, in 1831, to the industrial scale synthesis of sulphuric acid using a platinum catalyst.
In 1803, cerium was the first of thelanthanide metalsto be discovered, in Bastnäs, Sweden by Jöns Jakob Berzelius and Wilhelm Hisinger, and independently by Martin Heinrich Klaproth in Germany. The lanthanide metals were largely regarded as oddities until the 1960s when methods were developed to more efficiently separate them from one another. They have subsequently found uses in cell phones, magnets, lasers, lighting, batteries, catalytic converters, and in other applications enabling modern technologies.
Other metals discovered and prepared during this time were cobalt, nickel, manganese, molybdenum, tungsten, and chromium; and some of theplatinum groupmetals, palladium, osmium, iridium, and rhodium.
Light metals
All metals discovered until 1809 had relatively high densities; their heaviness was regarded as a singularly distinguishing criterion. From 1809 onward, light metals such as sodium, potassium, and strontium were isolated. Their low densities challenged conventional wisdom as to the nature of metals. They behaved chemically as metals however, and were subsequently recognized as such.
Aluminium was discovered in 1824 but it was not until 1886 that an industrial large-scale production method was developed. Prices of aluminium dropped and aluminium became widely used in jewelry, everyday items, eyeglass frames, optical instruments, tableware, and foil in the 1890s and early 20th century. Aluminium's ability to form hard yet light alloys with other metals provided the metal many uses at the time. During World War I, major governments demanded large shipments of aluminium for light strong airframes.
While pure metallic titanium (99.9%) was first prepared in 1910 it was not used outside the laboratory until 1932. In the 1950s and 1960s, the Soviet Union pioneered the use of titanium in military and submarine applications as part of programs related to the Cold War. Starting in the early 1950s, titanium came into use extensively in military aviation, particularly in high-performance jets, starting with aircraft such as theF-100 Super SabreandLockheed A-12andSR-71.
Metallic scandium was produced for the first time in 1937. The first pound of 99% pure scandium metal was produced in 1960. Production of aluminium-scandium alloys began in 1971 following a U.S. patent. Aluminium-scandium alloys were also developed in the USSR.
-
Chunks of sodium
-
Potassium pearls under paraffin oil. Size of the largest pearl is 0.5 cm.
-
Strontium crystals
-
Aluminium chunk,
2.6 grams,1 x 2 cm -
A bar of titanium crystals
-
Scandium, including a 1 cm3cube
The age of steel

The modern era insteelmakingbegan with the introduction ofHenry Bessemer'sBessemer processin 1855, the raw material for which was pig iron. His method let him produce steel in large quantities cheaply, thusmild steelcame to be used for most purposes for which wrought iron was formerly used. TheGilchrist-Thomas process(orbasic Bessemer process) was an improvement to the Bessemer process, made by lining the converter with abasicmaterial to remove phosphorus.
Due to its hightensile strengthand low cost, steel came to be a major component used inbuildings,infrastructure,tools,ships,automobiles,machines,appliances, andweapons.
In 1872, the Englishmen Clark and Woods patented an alloy that would today be considered astainless steel.The corrosion resistance of iron-chromium alloys had been recognized in 1821 by French metallurgistPierre Berthier.He noted their resistance against attack by some acids and suggested their use in cutlery. Metallurgists of the 19th century were unable to produce the combination of low carbon and high chromium found in most modern stainless steels, and the high-chromium alloys they could produce were too brittle to be practical. It was not until 1912 that the industrialization of stainless steel alloys occurred in England, Germany, and the United States.
The last stable metallic elements
By 1900 three metals withatomic numbersless than lead (#82), the heaviest stable metal, remained to be discovered: elements 71, 72, 75.
Von Welsbach,in 1906, proved that the old ytterbium also contained a new element (#71), which he namedcassiopeium.Urbainproved this simultaneously, but his samples were very impure and only contained trace quantities of the new element. Despite this, his chosen namelutetiumwas adopted.
In 1908, Ogawa found element 75 in thorianite but assigned it as element 43 instead of 75 and named itnipponium.In 1925 Walter Noddack, Ida Eva Tacke, and Otto Berg announced its separation from gadolinite and gave it the present name,rhenium.
Georges Urbain claimed to have found element 72 in rare-earth residues, while Vladimir Vernadsky independently found it in orthite. Neither claim was confirmed due to World War I, and neither could be confirmed later, as the chemistry they reported does not match that now known forhafnium.After the war, in 1922, Coster and Hevesy found it by X-ray spectroscopic analysis in Norwegian zircon.Hafniumwas thus the last stable element to be discovered, though rhenium was the last to be correctly recognized.
-
Lutetium, including a 1 cm3cube
-
Rhenium, including a 1 cm3cube
-
Hafnium, in the form of a 1.7 kg bar
By the end of World War II scientists had synthesized four post-uranium elements, all of which are radioactive (unstable) metals: neptunium (in 1940), plutonium (1940–41), and curium and americium (1944), representing elements 93 to 96. The first two of these were eventually found in nature as well. Curium and americium were by-products of the Manhattan project, which produced the world's first atomic bomb in 1945. The bomb was based on the nuclear fission of uranium, a metal first thought to have been discovered nearly 150 years earlier.
Post-World War II developments
Superalloys

Superalloys composed of combinations of Fe, Ni, Co, and Cr, and lesser amounts of W, Mo, Ta, Nb, Ti, and Al were developed shortly after World War II for use in high performance engines, operating at elevated temperatures (above 650 °C (1,200 °F)). They retain most of their strength under these conditions, for prolonged periods, and combine good low-temperature ductility with resistance to corrosion or oxidation. Superalloys can now be found in a wide range of applications including land, maritime, and aerospace turbines, and chemical and petroleum plants.
Transcurium metals
The successful development of the atomic bomb at the end of World War II sparked further efforts to synthesize new elements, nearly all of which are, or are expected to be, metals, and all of which are radioactive. It was not until 1949 that element 97 (Berkelium), next after element 96 (Curium), was synthesized by firing Alpha particles at an americium target. In 1952, element 100 (Fermium) was found in the debris of the first hydrogen bomb explosion; hydrogen, a nonmetal, had been identified as an element nearly 200 years earlier. Since 1952, elements 101 (Mendelevium) to 118 (Oganesson) have been synthesized.
Bulk metallic glasses

A metallic glass (also known as an amorphous or glassy metal) is a solid metallic material, usually an alloy, with a disordered atomic-scale structure. Most pure and alloyed metals, in their solid state, have atoms arranged in a highly ordered crystalline structure. They have a non-crystalline glass-like structure. But unlike common glasses, such as window glass, which are typically electrical insulators, amorphous metals have good electrical conductivity. Amorphous metals are produced in several ways, including extremely rapid cooling, physical vapor deposition, solid-state reaction, ion irradiation, and mechanical alloying. The first reported metallic glass was an alloy (Au75Si25) produced atCaltechin 1960. More recently, batches of amorphous steel with three times the strength of conventional steel alloys have been produced. Currently, the most important applications rely on the special magnetic properties of some ferromagnetic metallic glasses. The low magnetization loss is used in high-efficiency transformers. Theft control ID tags and other article surveillance schemes often use metallic glasses because of these magnetic properties.
Shape-memory alloys
A shape-memory alloy (SMA) is an alloy that "remembers" its original shape and when deformed returns to its pre-deformed shape when heated. While the shape memory effect had been first observed in 1932, in an Au-Cd alloy, it was not until 1962, with the accidental discovery of the effect in a Ni-Ti alloy that research began in earnest, and another ten years before commercial applications emerged. SMA's have applications in robotics and automotive, aerospace, and biomedical industries. There is another type of SMA, called a ferromagnetic shape-memory alloy (FSMA), that changes shape under strong magnetic fields. These materials are of particular interest as the magnetic response tends to be faster and more efficient than temperature-induced responses.
Quasicrystalline alloys
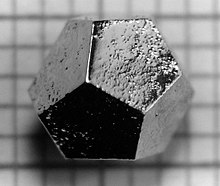
In 1984, Israeli chemist Dan Shechtman found an aluminum-manganese alloy having five-fold symmetry, in breach of crystallographic convention at the time which said that crystalline structures could only have two-, three-, four-, or six-fold symmetry. Due to fear of the scientific community's reaction, it took him two years to publish the results for which he was awarded the Nobel Prize in Chemistry in 2011. Since this time, hundreds of quasicrystals have been reported and confirmed. They exist in many metallic alloys (and some polymers). Quasicrystals are found most often in aluminum alloys (Al-Li-Cu, Al-Mn-Si, Al-Ni-Co, Al-Pd-Mn, Al-Cu-Fe, Al-Cu-V, etc.), but numerous other compositions are also known (Cd-Yb, Ti-Zr-Ni, Zn-Mg-Ho, Zn-Mg-Sc, In-Ag-Yb, Pd-U-Si, etc.).Quasicrystalseffectively have infinitely large unit cells.IcosahedriteAl63Cu24Fe13,the first quasicrystal found in nature, was discovered in 2009. Most quasicrystals have ceramic-like properties including low electrical conductivity (approaching values seen in insulators) and low thermal conductivity, high hardness, brittleness, and resistance to corrosion, and non-stick properties. Quasicrystals have been used to develop heat insulation, LEDs, diesel engines, and new materials that convert heat to electricity. New applications may take advantage of the low coefficient of friction and the hardness of some quasicrystalline materials, for example embedding particles in plastic to make strong, hard-wearing, low-friction plastic gears. Other potential applications include selective solar absorbers for power conversion, broad-wavelength reflectors, and bone repair and prostheses applications where biocompatibility, low friction, and corrosion resistance are required.
Complex metallic alloys
Complex metallic alloys (CMAs) are intermetallic compounds characterized by large unit cells comprising some tens up to thousands of atoms; the presence of well-defined clusters of atoms (frequently with icosahedral symmetry); and partial disorder within their crystalline lattices. They are composed of two or more metallic elements, sometimes with metalloids orchalcogenidesadded. They include, for example, NaCd2, with 348 sodium atoms and 768 cadmium atoms in the unit cell.Linus Paulingattempted to describe the structure of NaCd2in 1923, but did not succeed until 1955. At first called "giant unit cell crystals", interest in CMAs, as they came to be called, did not pick up until 2002, with the publication of a paper called "Structurally Complex Alloy Phases", given at the8th International Conference on Quasicrystals.Potential applications of CMAs include as heat insulation; solar heating; magnetic refrigerators; using waste heat to generate electricity; and coatings for turbine blades in military engines.
High-entropy alloys
High entropy alloys (HEAs) such as AlLiMgScTi are composed of equal or nearly equal quantities of five or more metals. Compared to conventional alloys with only one or two base metals, HEAs have considerably better strength-to-weight ratios, higher tensile strength, and greater resistance to fracturing, corrosion, and oxidation. Although HEAs were described as early as 1981, significant interest did not develop until the 2010s; they continue to be the focus of research in materials science and engineering because of their potential for desirable properties.
MAX phase
| MAX | M | A | X |
|---|---|---|---|
| Hf2SnC | Hf | Sn | C |
| Ti4AlN3 | Ti | Al | N |
| Ti3SiC2 | Ti | Si | C |
| Ti2AlC | Ti | Al | C |
| Cr2AlC2 | Cr | Al | C |
| Ti3AlC2 | Ti | Al | C |
In a Max phase,Mis an early transition metal,Ais an A group element (mostly group IIIA and IVA, or groups 13 and 14), andXis either carbon or nitrogen. Examples are Hf2SnC and Ti4AlN3.Such alloys have some of the best properties of metals and ceramics. These properties include high electrical and thermal conductivity, thermal shock resistance, damage tolerance, machinability, high elastic stiffness, and low thermal expansion coefficients.[74]They can be polished to a metallic luster because of their excellent electrical conductivities. During mechanical testing, it has been found that polycrystalline Ti3SiC2cylinders can be repeatedly compressed at room temperature, up to stresses of 1 GPa, and fully recover upon the removal of the load. Some MAX phases are also highly resistant to chemical attack (e.g. Ti3SiC2) and high-temperature oxidation in air (Ti2AlC, Cr2AlC2,and Ti3AlC2). Potential applications for MAX phase alloys include: as tough, machinable, thermal shock-resistant refractories; high-temperature heating elements; coatings for electrical contacts; and neutron irradiation resistant parts for nuclear applications.
See also
Note
- ^Trace elements having an abundance equalling or much less than one part per trillion (namelyTc,Pm,Po,At,Ra,Ac,Pa,Np,andPu) are not shown.
- ^In some cases, for example in the presence ofhigh energy gamma raysor in avery high temperature hydrogen rich environment,the subject nuclei may experience neutron loss or proton gain resulting in the production of (comparatively rare)neutron deficient isotopes.[54]
- ^The ejection of matter when two neutron stars collide is attributed to the interaction of theirtidal forces,possible crustal disruption, and shock heating (which is what happens if you floor the accelerator in car when the engine is cold).[57]
- ^Iron, cobalt, nickel, and tin are also siderophiles from a whole of Earth perspective.
- ^Another life-enabling role for iron is as a key constituent ofhemoglobin,which enables the transportation of oxygen from the lungs to the rest of the body.
- ^Bronzeis an alloy consisting primarily of copper, commonly with about 12% tin and often with the addition of other metals (such as aluminum, manganese, nickel, or zinc) and sometimes non-metals or metalloids such as arsenic, phosphorus, or silicon.
- ^In Damascus, Syria, blade-smiths forged knives and swords with a distinctive surface pattern composed of swirling patterns of light-etched regions on a nearly black background. These blades had legendary cutting abilities.The iron the smiths were usingwas sourced from India, and contained one or more carbide-forming elements, such as V, Mo, Cr, Mn, and Nb. Modern analysis of these weapons has shown that these elements supported the catalytic formation of carbon nanotubes, which in turn promoted the formation ofcementite(Fe3C) nanowires. The malleability of the carbon nanotubes offset the brittle nature of the cementite, and endowed the resulting steel with a unique combination of strength and flexibility. Knowledge of how to make what came to calledDamascus steeldied out in the eighteenth century possibly due to exhausting ore sources with the right combination of impurities. The techniques involved were not rediscovered until 2009.
- ^In ancient times, lead was regarded as the father of all metals.
- ^Paracelsus,a laterGerman Renaissancewriter, added the third principle of salt, carrying the nonvolatile and incombustible properties, in histria primadoctrine.These theories retained the four classical elements as underlying the composition of sulfur, mercury, and salt.
References
- ^abcdKittel, Charles (2018).Introduction to solid state physics.Paul McEuen (Global edition, [9th edition] ed.). Hoboken, NJ: Wiley.ISBN978-1-119-45416-8.
- ^abcdefghAshcroft, Neil W.; Mermin, N. David (1976).Solid state physics.New York: Holt, Rinehart and Winston.ISBN978-0-03-083993-1.
- ^abcdCallister, William D. (1997).Materials science and engineering: an introduction(4th ed.). New York: John Wiley & Sons.ISBN978-0-471-13459-6.
- ^Chiang, CK (1977). "Transport and optical properties of polythiazyl bromides: (SNBr0.4)x ".Solid State Communications.23(9): 607–612.Bibcode:1977SSCom..23..607C.doi:10.1016/0038-1098(77)90530-0.;Greenwood, NN; Earnshaw, A (1998).Chemistry of the Elements.Oxford: Butterworth-Heinemann. p. 727.ISBN978-0-7506-3365-9.;Mutlu, H; Theato, P (2021). "Polymers with sulfur-nitrogen bonds". In Zhang, X; Theato, P (eds.).Sulfur-Containing Polymers: From Synthesis to Functional Materials.Weinheim: Wiley-VCH. pp. 191–234 (191).ISBN978-3-527-34670-7.
- ^Yonezawa, F. (2017).Physics of Metal-Nonmetal Transitions.Amsterdam: IOS Press. p. 257.ISBN978-1-61499-786-3.
SirNevill Mott(1905–1996) wrote a letter to a fellow physicist,Prof. Peter P. Edwards,in which he notes... I've thought a lot about 'What is a metal?' and I think one can only answer the question atT= 0 (the absolute zero of temperature). There a metal conducts and a nonmetal doesn't.
- ^Martin, John C."What we learn from a star's metal content".John C. Martin's Homepage.RetrievedMarch 25,2021.
- ^Martin, John C.; Morrison, Heather L. (May 18, 1998) [1998]."A New Analysis of RR Lyrae Kinematics in the Solar Neighborhood".The Astronomical Journal.116(4) (published October 1, 1998): 1724–1735.arXiv:astro-ph/9806258.Bibcode:1998AJ....116.1724M.doi:10.1086/300568.S2CID18530430– via IOPscience.
- ^Roe, J.; Roe, M. (1992). "World's coinage uses 24 chemical elements".World Coinage News.19(4, 5): 24–25, 18–19.
- ^abcdStampfl, C.; Mannstadt, W.; Asahi, R.; Freeman, A. J. (2001)."Electronic structure and physical properties of early transition metal mononitrides: Density-functional theory LDA, GGA, and screened-exchange LDA FLAPW calculations".Physical Review B.63(15): 155106.Bibcode:2001PhRvB..63o5106S.doi:10.1103/PhysRevB.63.155106.
- ^abcMortimer, Charles E. (1975).Chemistry: A Conceptual Approach(3rd ed.). New York: D. Van Nostrad Company.
- ^Moller, P.; Nix, J. R. (1994).Fission properties of the heaviest elements(PDF).Dai 2 Kai Hadoron Tataikei no Simulation Symposium, Tokai-mura, Ibaraki, Japan.University of North Texas.Retrieved2020-02-16.
- ^abcWeertman, Johannes; Weertman, Julia R. (1992).Elementary dislocation theory.New York: Oxford University Press.ISBN978-0-19-506900-6.
- ^Timoshenko, Stephen (1983-01-01).History of Strength of Materials: With a Brief Account of the History of Theory of Elasticity and Theory of Structures.Courier Corporation.ISBN978-0-486-61187-7.
- ^Holleman, A. F.; Wiberg, E. (2001).Inorganic Chemistry.San Diego: Academic Press.ISBN0-12-352651-5.
- ^Koster, G. (2015).Epitaxial growth of complex metal oxides.Boston, MA: Elsevier.ISBN978-1-78242-245-7.
- ^Schiff, Leonard(1959).Quantum Mechanics(PDF).McGraw-Hill.
- ^abSolymar, L.; Walsh, D. (2004).Electrical properties of materials(7th ed.). Oxford; New York: Oxford University Press.ISBN978-0-19-926793-4.
- ^Slonczewski, J. C.; Weiss, P. R. (1958)."Band Structure of Graphite".Physical Review.109(2): 272–279.doi:10.1103/PhysRev.109.272.ISSN0031-899X.
- ^Hecker, Siegfried S. (2000)."Plutonium and its alloys: from atoms to microstructure"(PDF).Los Alamos Science.26:290–335.Archived(PDF)from the original on February 24, 2009.RetrievedFebruary 15,2009.
- ^Koester, D; Chanmugam, G (1990)."Physics of white dwarf stars".Reports on Progress in Physics.53(7): 837–915.doi:10.1088/0034-4885/53/7/001.ISSN0034-4885.
- ^Skośkiewicz, T. (2005). "Thermal Conductivity at Low Temperatures".Encyclopedia of Condensed Matter Physics.Elsevier. pp. 159–164.doi:10.1016/b0-12-369401-9/01168-2.ISBN978-0-12-369401-0.
- ^Bockris, J. O'M; Reddy, Amulya K. N. (1977).Modern electrochemistry. 2(3. print ed.). New York: Plenum Pr.ISBN978-0-306-25002-6.
- ^Kelly, Robert G.; Scully, John R.; Shoesmith, David; Buchheit, Rudolph G. (2002-09-13).Electrochemical Techniques in Corrosion Science and Engineering(0 ed.). CRC Press.doi:10.1201/9780203909133.ISBN978-0-203-90913-3.
- ^Greenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.ISBN978-0-08-037941-8.
- ^abSiekierski, S.; Burgess, J. (2002).Concise Chemistry of the Elements.Horwood. pp. 60–66.ISBN978-1-898563-71-6.
- ^Mewes, Jan-Michael; Smits, Odile Rosette; Jerabek, Paul; Schwerdtfeger, Peter (25 July 2019)."Oganesson is a Semiconductor: On the Relativistic Band-Gap Narrowing in the Heaviest Noble-Gas Solids".Angewandte Chemie.58(40): 14260–14264.doi:10.1002/anie.201908327.PMC6790653.PMID31343819.
- ^Jang, J. M.; Lee, H. S.; Singh, J. K. (December 17, 2020)."Electromagnetic Shielding Performance of Different Metallic Coatings Deposited by Arc Thermal Spray Process".Materials.13(24): 5776.Bibcode:2020Mate...13.5776J.doi:10.3390/ma13245776.PMC7767199.PMID33348891.
- ^"Metals Program Overview"(PDF).arpa-e.energy.gov.RetrievedJune 4,2024.
- ^Russell, A. M.; Lee, K. L. (2005).Structure–Property Relations in Nonferrous Metals.Hoboken, NJ: John Wiley & Sons. pp. passim.Bibcode:2005srnm.book.....R.ISBN978-0-471-64952-6.
- ^Introduction to Dislocations.Elsevier. 2001.doi:10.1016/b978-0-7506-4681-9.x5000-7.ISBN978-0-7506-4681-9.
- ^"Belmont Metals - White Metals".Belmont Metals.2019-04-17.Retrieved2024-07-08.
- ^Roden, Arabella (2019-11-04)."A closer look at the world of white metals".jewellermagazine.Retrieved2024-07-08.
- ^Prsctical guidance in relation to the hallmarking act 1973(PDF).Assay offices of Great Britain.
- ^Metal contamination.Editions Quae. 2006.ISBN978-2-7592-0011-5.
- ^Tunay, Olcay; Kabdasli, Isik; Arslan-Alaton, Idil; Olmez-Hanci, Tugba (2010).Chemical Oxidation Applications for Industrial Wastewaters.IWA Publishing.ISBN978-1-84339-307-8.
- ^Walther, John V. (2013).Earth's Natural Resources.Jones & Bartlett Publishers.ISBN978-1-4496-3234-2.
- ^Abdul-Rahman, Yahia (2014).The Art of RF (Riba-Free) Islamic Banking and Finance: Tools and Techniques for Community-Based Banking.John Wiley & Sons.ISBN978-1-118-77096-2.
- ^van Hove, Ruud P.; Sierevelt, Inger N.; van Royen, Barend J.; Nolte, Peter A. (2015)."Titanium-Nitride Coating of Orthopaedic Implants: A Review of the Literature".BioMed Research International.2015:1–9.doi:10.1155/2015/485975.ISSN2314-6133.PMC4637053.PMID26583113.
- ^Santecchia, Eleonora; Hamouda, A. M. S.; Musharavati, Farayi; Zalnezhad, Erfan; Cabibbo, Marcello; Spigarelli, Stefano (2015)."Wear resistance investigation of titanium nitride-based coatings".Ceramics International.41(9, Part A): 10349–10379.doi:10.1016/j.ceramint.2015.04.152.ISSN0272-8842.
- ^Matthews, A. (1985)."Titanium Nitride PVD Coating Technology".Surface Engineering.1(2): 93–104.doi:10.1179/sur.1985.1.2.93.ISSN0267-0844.
- ^K, Namsheer; Rout, Chandra Sekkha (2021)."Conducting polymers: a comprehensive review on recent advances in synthesis, properties and applications".RSC Advances.11(10): 5659–5697.Bibcode:2021RSCAd..11.5659K.doi:10.1039/D0RA07800J.PMC9133880.PMID35686160.
- ^Das, Tapan K.; Prusty, Smita (2012)."Review on Conducting Polymers and Their Applications".Polymer-Plastics Technology and Engineering.51(14): 1487–1500.doi:10.1080/03602559.2012.710697.ISSN0360-2559.
- ^Swager, Timothy M. (2017)."50th Anniversary Perspective: Conducting/Semiconducting Conjugated Polymers. A Personal Perspective on the Past and the Future".Macromolecules.50(13): 4867–4886.Bibcode:2017MaMol..50.4867S.doi:10.1021/acs.macromol.7b00582.ISSN0024-9297.
- ^Beygisangchin, Mahnoush; Abdul Rashid, Suraya; Shafie, Suhaidi; Sadrolhosseini, Amir Reza; Lim, Hong Ngee (2021-06-18)."Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review".Polymers.13(12): 2003.doi:10.3390/polym13122003.ISSN2073-4360.PMC8234317.PMID34207392.
- ^de Groot, R. A.; Mueller, F. M.; Engen, P. G. van; Buschow, K. H. J. (1983-06-20)."New Class of Materials: Half-Metallic Ferromagnets".Physical Review Letters.50(25): 2024–2027.doi:10.1103/PhysRevLett.50.2024.ISSN0031-9007.
- ^Coey, J. M. D.; Venkatesan, M. (2002-05-15)."Half-metallic ferromagnetism: Example of CrO2 (invited)".Journal of Applied Physics.91(10): 8345–8350.doi:10.1063/1.1447879.ISSN0021-8979.
- ^Armitage, N. P.; Mele, E. J.; Vishwanath, Ashvin (2018-01-22)."Weyl and Dirac semimetals in three-dimensional solids".Reviews of Modern Physics.90(1).doi:10.1103/RevModPhys.90.015001.ISSN0034-6861.
- ^Wang, Yang; N. Mansour; A. Salem; K.F. Brennan & P.P. Ruden (1992). "Theoretical study of a potential low-noise semimetal-based avalanche photodetector".IEEE Journal of Quantum Electronics.28(2): 507–513.Bibcode:1992IJQE...28..507W.doi:10.1109/3.123280.
- ^Bubnova, Olga; Zia, Ullah Khan; Wang, Hui (2014)."Semi-Metallic Polymers".Nature Materials.13(2): 190–4.Bibcode:2014NatMa..13..190B.doi:10.1038/nmat3824.PMID24317188.S2CID205409397.
- ^abCox 1997,pp. 73–89
- ^Cox 1997,pp. 32, 63, 85
- ^Podosek 2011,p. 482
- ^Padmanabhan 2001,p. 234
- ^Rehder 2010,pp. 32, 33
- ^Hofmann 2002,pp. 23–24
- ^Hadhazy 2016
- ^Choptuik, Lehner & Pretorias 2015,p. 383
- ^Cox 1997,pp. 83, 91, 102–103
- ^"Los Alamos National Laboratory – Sodium".Retrieved2007-06-08.
- ^"Los Alamos National Laboratory – Aluminum".Retrieved2007-06-08.
- ^abThe Recycling Rates of Metals: A Status ReportArchived2016-01-01 at theWayback Machine2010,International Resource Panel,United Nations Environment Programme
- ^Tread lightly: Aluminium attackCarolyn Fry, Guardian.co.uk, 22 February 2008.
- ^Metal Stocks in Society: Scientific SynthesisArchived2016-01-01 at theWayback Machine2010,International Resource Panel,United Nations Environment Programme
- ^Reardon, Arthur C. (2011).Metallurgy for the non-metallurgist.Materials Park, Ohio: ASM International. pp. 73–84.ISBN978-1-61503-845-9.OCLC780082219.
- ^ Tylecote, R. F. (1992).A History of Metallurgy, Second Edition.London: Maney Publishing, for the Institute of Materials.ISBN978-1-902653-79-2.Archived fromthe originalon 2015-04-02.
- ^Thornton, C.; Lamberg-Karlovsky, C. C.; Liezers, M.; Young, S. M. M. (2002). "On pins and needles: tracing the evolution of copper-based alloying at Tepe Yahya, Iran, via ICP-MS analysis of Common-place items".Journal of Archaeological Science.29(12): 1451–1460.Bibcode:2002JArSc..29.1451T.doi:10.1006/jasc.2002.0809.
- ^Kaufman, Brett. "Metallurgy and Archaeological Change in the Ancient Near East".Backdirt: Annual Review.2011:86.
- ^Akanuma, H. (2005). "The significance of the composition of excavated iron fragments taken from Stratum III at the site of Kaman-Kalehöyük, Turkey".Anatolian Archaeological Studies.14.Tokyo: Japanese Institute of Anatolian Archaeology: 147–158.
- ^"Ironware piece unearthed from Turkey found to be oldest steel".The Hindu.Chennai, India. 2009-03-26. Archived fromthe originalon 2009-03-29.Retrieved2009-03-27.
- ^Gabriel, RA (1990).The Culture of War: Invention and Early Development.Westport CT: Greenwood Publishing Group. p. 108.ISBN978-0-313-26664-5.
- ^Knauth, P. (1976).The Metalsmiths, revised edition.London: Time-Life International. pp. 133, 137.
- ^Published inThe Delineator,Sept. 1909. Reprinted as the introduction toRewards and Fairiesin 1910.
- ^Georgius Agricola,De Re Metallica(1556) Tr. Herbert Clark Hoover & Lou Henry Hoover (1912); Footnote quotingDe Natura Fossilium(1546), p. 180
- ^Hanaor, D.A.H.; Hu, L.; Kan, W.H.; Proust, G.; Foley, M.; Karaman, I.; Radovic, M. (2016)."Compressive performance and crack propagation in Al alloy/Ti2AlC composites".Materials Science and Engineering: A.672:247–256.arXiv:1908.08757.doi:10.1016/j.msea.2016.06.073.
Further reading
- Choptuik M. W.,Lehner L. & Pretorias F. 2015, "Probing strong-field gravity through numerical simulation", inA. Ashtekar,B. K. Berger,J. Isenberg & M. MacCallum (eds),General Relativity and Gravitation: A Centennial Perspective,Cambridge University Press, Cambridge,ISBN978-1-107-03731-1.
- Cox, P. A. (1997).The elements: Their origin, abundance and distribution.Oxford: Oxford University Press.ISBN978-0-19-855298-7.
- Crow J. M. 2016, "Impossible alloys: How to make never-before-seen metals",New Scientist,12 October
- Hadhazy A. 2016, "Galactic 'Gold Mine' Explains the Origin of Nature's Heaviest Elements",Science Spotlights,10 May 2016, accessed 11 July 2016.
- Hofmann S. 2002,On Beyond Uranium: Journey to the End of the Periodic Table,Taylor & Francis,London,ISBN978-0-415-28495-0.
- Padmanabhan T.2001,Theoretical Astrophysics,vol. 2, Stars and Stellar Systems,Cambridge University Press,Cambridge,ISBN978-0-521-56241-6.
- Parish R. V. 1977,The metallic elements,Longman, London,ISBN978-0-582-44278-8
- Podosek F. A. 2011, "Noble gases", in H. D. Holland &K. K. Turekian(eds),Isotope Geochemistry: From the Treatise on Geochemistry,Elsevier, Amsterdam, pp. 467–492,ISBN978-0-08-096710-3.
- Raymond R. 1984,Out of the fiery furnace: The impact of metals on the history of mankind,Macmillan Australia, Melbourne,ISBN978-0-333-38024-6
- Rehder D. 2010,Chemistry in Space: From Interstellar Matter to the Origin of Life,Wiley-VCH, Weinheim,ISBN978-3-527-32689-1.
- Russell A. M. & Lee K. L. 2005,Structure–property relations in nonferrous metals,John Wiley & Sons, Hoboken, New Jersey,ISBN978-0-471-64952-6
- Street A. & Alexander W. 1998,Metals in the service of man,11thed., Penguin Books, London,ISBN978-0-14-025776-2
- Wilson A. J. 1994,The living rock: The story of metals since earliest times and their impact on developing civilization,Woodhead Publishing, Cambridge,ISBN978-1-85573-154-7
External links
- Official websiteof ASM International (formerly the American Society for Metals)
- Official websiteof The Minerals, Metals & Materials Society