Methyl formate
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methyl formate | |||
| Systematic IUPAC name
Methyl methanoate | |||
| Other names
R-611
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.166 | ||
| EC Number |
| ||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H4O2 | |||
| Molar mass | 60.052g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | pleasant[1] | ||
| Density | 0.98 g/cm3 | ||
| Melting point | −100 °C (−148 °F; 173 K) | ||
| Boiling point | 32 °C (90 °F; 305 K) | ||
| 30% (20°C)[1] | |||
| Vapor pressure | 634 hPa (476 mmHg) (20°C)[1] | ||
| -32.0·10−6cm3/mol | |||
| Hazards | |||
| GHSlabelling:[3] | |||
 
| |||
| Danger | |||
| H224,H302,H319,H332,H335 | |||
| P210,P233,P240,P241,P242,P243,P261,P264,P270,P271,P280,P301+P312,P303+P361+P353,P304+P312,P304+P340,P305+P351+P338,P312,P330,P337+P313,P370+P378,P403+P233,P403+P235,P405,P501 | |||
| NFPA 704(fire diamond) | |||
| Flash point | −19 °C; −2 °F; 254 K[1] | ||
| Explosive limits | 4.5%-23%[1] | ||
| Lethal doseor concentration (LD, LC): | |||
LD50(median dose)
|
1622 mg/kg (oral, rabbit)[2] | ||
LCLo(lowest published)
|
50,000 ppm (guinea pig, 20 min)[2] | ||
| NIOSH(US health exposure limits): | |||
PEL(Permissible)
|
TWA 100 ppm (250 mg/m3)[1] | ||
REL(Recommended)
|
TWA 100 ppm (250 mg/m3) ST 150 ppm (375 mg/m3)[1] | ||
IDLH(Immediate danger)
|
4500 ppm[1] | ||
| Safety data sheet(SDS) | Oxford MSDS | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Methyl formate,also calledmethyl methanoate,is the methylesterofformic acid.The simplest example of a carboxylate ester, it is a colorless liquid with an ethereal odour, highvapor pressure,and lowsurface tension.It is a precursor to many other compounds of commercial interest.[4]
Production[edit]
In the laboratory, methyl formate can be produced by thecondensation reactionofmethanolandformic acid,as follows:
- HCOOH + CH3OH → HCOOCH3+ H2O
Industrial methyl formate, however, is usually produced by the combination ofmethanolandcarbon monoxide(carbonylation) in the presence of a strong base, such assodium methoxide:[4]
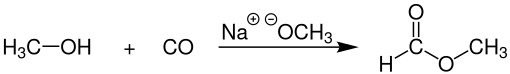
This process, practiced commercially by BASF among other companies gives 96% selectivity toward methyl formate. Thecatalystfor this process is sensitive to water, which can be present in thecarbon monoxidefeedstock, which is commonly derived fromsynthesis gas.Very drycarbon monoxideis, therefore, essential.[5]
Uses[edit]
Methyl formate is used primarily to manufactureformamide,dimethylformamide,andformic acid.These compounds are precursors or building blocks for many useful derivatives.
Because of its highvapor pressure,it is used for quick-drying finishes and as ablowing agentfor somepolyurethane foamapplications (for exampleEcomate®manufactured by Foam Supplies Inc.) and as a replacement forCFCs,HCFCs,andHFCs.Methyl formate has zeroozone depletion potentialand zeroglobal warming potential[citation needed].It is also used as aninsecticide.
A historical use of methyl formate, which sometimes brings it attention, was in refrigeration. Before the introduction of less-toxic refrigerants, methyl formate was used as an alternative tosulfur dioxidein domestic refrigerators, such as some models of the famous GE Monitor Top.
References[edit]
- ^abcdefghNIOSH Pocket Guide to Chemical Hazards."#0417".National Institute for Occupational Safety and Health(NIOSH).
- ^ab"Methyl formate".Immediately Dangerous to Life or Health Concentrations (IDLH).National Institute for Occupational Safety and Health(NIOSH).
- ^"Methyl formate".pubchem.ncbi.nlm.nih.gov.Retrieved19 December2021.
- ^abWerner Reutemann and Heinz Kieczka "Formic Acid" inUllmann's Encyclopedia of Industrial Chemistry2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a12_013
- ^W. Couteau, J. Ramioulle, US Patent US4216339