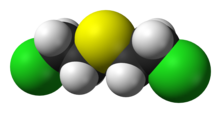
Mustard gas

| |

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloro-2-[(2-chloroethyl)sulfanyl]ethane | |
| Other names
Bis(2-chloroethyl) sulfide
HD Iprit Schwefel-LOST Lost Sulfur mustard Senfgas Yellow cross liquid Yperite Distilled mustard Mustard T- mixture 1,1'-thiobis[2-chloroethane] Dichlorodiethyl sulfide | |
| Identifiers | |
3D model (JSmol)
|
|
| 1733595 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.209.973 |
| EC Number |
|
| 324535 | |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C4H8Cl2S | |
| Molar mass | 159.07g·mol−1 |
| Appearance | Colorless if pure. Normally ranges from pale yellow to dark brown. Slight garlic or horseradish type odor.[1] |
| Density | 1.27 g/mL, liquid |
| Melting point | 14.45 °C (58.01 °F; 287.60 K) |
| Boiling point | 217 °C (423 °F; 490 K) begins to decompose at 217 °C (423 °F) and boils at 218 °C (424 °F) |
| 7.6 mg/L at 20°C[2] | |
| Solubility | Alcohols,ethers,hydrocarbons,lipids,THF |
| Hazards | |
| Occupational safety and health(OHS/OSH): | |
Main hazards
|
Flammable, toxic, vesicant, carcinogenic, mutagenic |
| GHSlabelling:[3] | |
 
| |
| Danger | |
| H300,H310,H315,H319,H330,H335 | |
| P260,P261,P262,P264,P270,P271,P280,P284,P301+P310,P302+P350,P302+P352,P304+P340,P305+P351+P338,P310,P312,P320,P321,P322,P330,P332+P313,P337+P313,P361,P362,P363,P403+P233,P405,P501 | |
| NFPA 704(fire diamond) | |
| Flash point | 105 °C (221 °F; 378 K) |
| Safety data sheet(SDS) | External MSDS |
| Related compounds | |
Related compounds
|
Nitrogen mustard,Bis(chloroethyl) ether,Chloromethyl methyl sulfide |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Mustard gasorsulfur mustardare names commonly used for theorganosulfurchemical compoundbis(2-chloroethyl) sulfide,which has the chemical structure S(CH2CH2Cl)2,as well as other species. In the wider sense, compounds with the substituents−SCH2CH2X or −N(CH2CH2X)2are known assulfur mustardsornitrogen mustards,respectively, where X = Cl or Br. Such compounds are potentalkylating agents,which can interfere with several biological processes. Also known as mustard agents, this family of compounds comprises infamouscytotoxinsandblister agentswith a long history of use aschemical weapons.The namemustard gasis technically incorrect; the substances, whendispersed,are often not gases but a fine mist of liquid droplets.[4]Sulfur mustards are viscous liquids at room temperature and have an odor resemblingmustard plants,garlic,orhorseradish,hence the name.[4]When pure, they are colorless, but when used in impure forms, such as in warfare, they are usuallyyellow-brown.Mustard gases formblisterson exposed skin and in the lungs, often resulting in prolonged illness ending in death.
History as chemical weapons[edit]
Sulfur mustard is a type of chemical warfare agent.[5]As a chemical weapon, mustard gas was first used inWorld War I,and has been used in several armed conflicts since then, including theIran–Iraq War,resulting in more than 100,000 casualties.[6][7]Today, sulfur-based andnitrogen-basedmustard agents are regulated underSchedule 1of the 1993Chemical Weapons Convention,as substances with few uses other than inchemical warfare(though since then, mustard gas has been found to be useful in cancerchemotherapy[8]). Mustard agents can be deployed by means ofartillery shells,aerial bombs,rockets,or by spraying from aircraft.
Mechanism of cellular toxicity[edit]
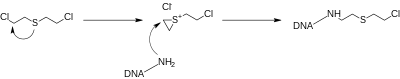
Sulfur mustards readily eliminatechlorideions by intramolecularnucleophilic substitutionto form cyclicsulfoniumions. These very reactive intermediates tend to permanentlyalkylatenucleotidesinDNAstrands, which can prevent cellular division, leading toprogrammed cell death.[2]Alternatively, if cell death is not immediate, the damaged DNA can lead to the development of cancer.[2]Oxidative stresswould be another pathology involved in mustard gas toxicity.
In the wider sense, compounds with the structural element BC2H4X, where X is anyleaving groupand B is aLewis base,are known asmustards.[citation needed]Such compounds can form cyclic"onium" ions(sulfonium,ammonium,etc.) that are goodalkylating agents.Other such compounds are bis(2-haloethyl)ethers (oxygen mustards), the (2-haloethyl)amines (nitrogen mustards), andsesquimustard,which has two α-chloroethyl thioether groups (ClC2H4S−) connected by an ethylene bridge (−C2H4−).[citation needed]These compounds have a similar ability to alkylate DNA, but their physical properties vary.
Physiological effects[edit]
Mustard gases react withDNA,which interferes with cellular division and can lead to mutations.[2]
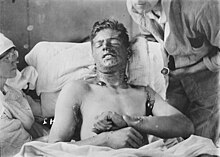
Mustard gases are extremely toxic and have powerfulblisteringeffects on victims. Their alkylating capabilities make them stronglycarcinogenicandmutagenic.Furthermore, they are highlylipophilic,which accelerates their absorption into the body.[2]Because people exposed to mustard agents rarely suffer immediate symptoms, and contaminated areas may appear completely normal, victims can unknowingly receive high doses. Within 24 hours of exposure, they experience intenseitchingand skin irritation. If this irritation goes untreated, blisters filled withpuscan form wherever the agent contacted the skin. These arechemical burnsand are very debilitating. Mustard gases easily penetrate clothing fabrics such as wool or cotton, so it is not only exposed skin that gets burned. If the victim's eyes were exposed, then they become sore, starting withconjunctivitis(also known as pink eye), after which the eyelids swell, resulting in temporary blindness. Extreme ocular exposure to mustard gas vapors may result incorneal ulceration,anterior chamber scarring, andneovascularization.[9][10][11][12]In these severe and infrequent cases,corneal transplantationhas been used as a treatment option.[13]Miosis,when the pupil constricts more than usual, may also occur, which is probably the result of the cholinomimetic activity of mustard.[14]At very high concentrations, if inhaled, mustard agents cause bleeding and blistering within therespiratory system,damagingmucous membranesand causingpulmonary edema.Depending on the level of contamination, mustard agent burns can vary betweenfirstandsecond degree burns,though they can also be every bit as severe, disfiguring, and dangerous asthird degree burns.[15]Burns that are severe (i.e. covering more than 50% of the victim's skin) are often fatal, with death occurring after only days or weeks. Mild or moderate exposure to mustard gases is unlikely to kill, though victims still require lengthy periods of medical treatment andconvalescencebefore recovery is complete.
Mustard gases' carcinogenic and mutagenic effects mean that victims, even if they fully recover, have an increased risk of developingcancerlater in life. In a study of patients 25 years after wartime exposure to chemical weaponry, c-DNA microarray profiling indicated that 122 genes were significantly mutated in the lungs and airways of mustard gas victims. Those genes all correspond to functions commonly affected by mustard gas exposure, includingapoptosis,inflammation, and stress responses.[16]The long-term ocular complications include burning, tearing, itching,photophobia,presbyopia,pain, and foreign-body sensations.[17][18]

Mustard gases' blistering effects can be neutralized byoxidationorchlorination,using household bleach (sodium hypochlorite), or by nucleophilic attack using decontamination solutions such as "DS2" (2%NaOH,70%diethylenetriamine,28%2-methoxyethanol). After initial decontamination of the victim's wounds is complete, medical treatment is similar to that required by any conventional burn. The degree of pain and discomfort suffered by the victim is also comparable. Mustard agent burns do not heal quickly, and (as with other types of burns) present a risk ofsepsiscaused bypathogenssuch asStaphylococcus aureusandPseudomonas aeruginosa.The mechanisms behind mustard gas's effect on endothelial cells are still being studied, but recent studies have shown that high levels of exposure can induce high rates of bothnecrosisandapoptosis.In vitro tests have shown that at low concentrations of mustard gas, where apoptosis is the predominant result of exposure, pretreatment with 50 mMN-acetyl-L-cysteine(NAC) was able to decrease the rate of apoptosis. NAC protectsactinfilaments from reorganization by mustard gas, demonstrating that actin filaments play a large role in the severe burns observed in victims.[19]
A British nurse treating soldiers with mustard agent burns duringWorld War Icommented:[20]
They cannot be bandaged or touched. We cover them with a tent of propped-up sheets. Gas burns must be agonizing because usually the other cases do not complain, even with the worst wounds, but gas cases are invariably beyond endurance and they cannot help crying out.
Formulations[edit]

In its history, various types and mixtures of mustard gas have been employed. These include:
- H– Also known asHS( "Hun Stuff" ) orLevinstein mustard.This is named after the inventor of the "quick but dirty"Levinstein Processfor manufacture,[21][22]reacting dryethylenewithdisulfur dichlorideunder controlled conditions. Undistilled mustard gas contains 20–30% impurities, which means it does not store as well as HD. Also, as it decomposes, it increases invapor pressure,making the munition it is contained in likely to split, especially along a seam, releasing the agent to the atmosphere.[1]
- HD– CodenamedPyroby the British, andDistilled Mustardby the US.[1]Distilledmustard of 95% or higher purity. The term "mustard gas" usually refers to this variety of mustard.
- HT– CodenamedRuncolby the British, andMustard T- mixtureby the US.[1]A mixture of 60% HD mustard and 40%O-mustard,a related vesicant with lowerfreezing point,lowervolatilityand similar vesicant characteristics.
- HL– A blend of distilled mustard (HD) andlewisite(L), originally intended for use in winter conditions due to its lower freezing point compared to the pure substances. The lewisite component of HL was used as a form ofantifreeze.[23]
- HQ– A blend of distilled mustard (HD) and sesquimustard (Q) (Gates and Moore 1946).
- Yellow Cross– any of several blends containing sulfur mustard and sometimes arsine agents, along with solvents and other additives.
Commonly-stockpiled mustard agents (class)[edit]
| Chemical | Code | Trivial name | CAS number | PubChem | Structure |
|---|---|---|---|---|---|
| Bis(2-chloroethyl) sulfide | H, HD | Mustard | 505-60-2 | CID 10461fromPubChem | |
| 1,2-Bis(2-chloroethylsulfanyl) ethane | Q | Sesquimustard | 3563-36-8 | CID 19092fromPubChem | |
| 2-Chloroethyl ethyl sulfide | Half mustard | 693-07-2 | CID 12733fromPubChem | ||
| Bis(2-(2-chloroethylsulfanyl)ethyl) ether | T | O-mustard | 63918-89-8 | CID 45452fromPubChem | |
| 2-Chloroethyl chloromethyl sulfide | 2625-76-5 | ||||
| Bis(2-chloroethylsulfanyl) methane | HK | 63869-13-6 | |||
| 1,3-Bis(2-chloroethylsulfanyl) propane | 63905-10-2 | ||||
| 1,4-Bis(2-chloroethylsulfanyl) butane | 142868-93-7 | ||||
| 1,5-Bis(2-chloroethylsulfanyl) pentane | 142868-94-8 | ||||
| Bis((2-chloroethylsulfanyl)methyl) ether | 63918-90-1 |
History[edit]
Development[edit]
Mustard gases were possibly developed as early as 1822 byCésar-Mansuète Despretz(1798–1863).[24]Despretz described the reaction ofsulfur dichlorideandethylenebut never made mention of any irritating properties of the reaction product. In 1854, another French chemist, Alfred Riche (1829–1908), repeated this procedure, also without describing any adverse physiological properties. In 1860, the British scientistFrederick Guthriesynthesized and characterized the mustard agent compound and noted its irritating properties, especially in tasting.[25]Also in 1860, chemistAlbert Niemann,known as a pioneer incocainechemistry, repeated the reaction, and recorded blister-forming properties. In 1886,Viktor Meyerpublished a paper describing a synthesis that produced good yields. He combined2-chloroethanolwithaqueouspotassium sulfide,and then treated the resultingthiodiglycolwithphosphorus trichloride.The purity of this compound was much higher and consequently the adverse health effects upon exposure were much more severe. These symptoms presented themselves in his assistant, and in order to rule out the possibility that his assistant was suffering from a mental illness (psychosomatic symptoms), Meyer had this compound tested on laboratoryrabbits,most of which died. In 1913, the English chemistHans Thacher Clarke(known for theEschweiler-Clarke reaction) replaced the phosphorus trichloride withhydrochloric acidin Meyer's formulation while working withEmil FischerinBerlin.Clarke was hospitalized for two months for burns after one of his flasks broke. According to Meyer, Fischer's report on this accident to theGerman Chemical Societysent theGerman Empireon the road to chemical weapons.[26]
Mustard gas can have the effect of turning a patient's skin different colors, including shades of red, orange, pink, and in unusual cases, blue. TheGerman EmpireduringWorld War Irelied on the Meyer-Clarke method because2-chloroethanolwas readily available from the German dye industry of that time.
Use[edit]

Mustard gas was firstused in World War Iby the German army against British and Canadian soldiers nearYpres,Belgium, on July 12, 1917.[27]Later also against theFrench Second Army.Yperiteis "a name used by the French, because the compound was first used at Ypres."[28]The Allies did not use mustard gas until November 1917 atCambrai,France, after the armies had captured a stockpile of German mustard shells. It took the British more than a year to develop their own mustard agent weapon, with production of the chemicals centred onAvonmouth Docks(the only option available to the British was the Despretz–Niemann–Guthrie process).[29][30]This was used first in September 1918 during the breaking of theHindenburg Line.
Mustard gas was originally assigned the name LOST, after the scientists Wilhelm Lommel andWilhelm Steinkopf,who developed a method of large-scale production for theImperial German Armyin 1916.[31]
Mustard gas was dispersed as anaerosolin a mixture with other chemicals, giving it a yellow-brown color. Mustard agent has also been dispersed in such munitions asaerial bombs,land mines,mortar rounds,artillery shells,androckets.[1]Exposure to mustard agent was lethal in about 1% of cases. Its effectiveness was as anincapacitating agent.The early countermeasures against mustard agent were relatively ineffective, since a soldier wearing agas maskwas not protected against absorbing it through his skin and being blistered. A common countermeasure was using a urine-soaked mask or facecloth to prevent or reduce injury, a readily available remedy attested by soldiers in documentaries (e.g.They Shall Not Grow Oldin 2018) and others (such as forward aid nurses) interviewed between 1947 and 1981 by the British Broadcasting Corporation for various World War One history programs; however, the effectiveness of this measure is unclear.
Mustard gas can remain in the ground for weeks, and it continues to cause ill effects. If mustard agent contaminates one's clothing and equipment while cold, then other people with whom they share an enclosed space could become poisoned as contaminated items warm up enough material to become an airborne toxic agent. An example of this was depicted in a British and Canadian documentary about life in the trenches, particularly once the "sousterrain" (subways and berthing areas underground) were completed in Belgium and France. Towards the end of World War I, mustard agent was used in high concentrations as anarea-denial weaponthat forced troops to abandon heavily contaminated areas.

Since World War I, mustard gas has been used in several wars and other conflicts, usually against people who cannot retaliate in kind:[32]
- United Kingdom against theRed Armyin 1919[33]
- Alleged British use in Mesopotamia in 1920[34]
- Spainagainst theRifianresistance inMoroccoduring theRif Warof 1921–27 (see also:Spanish use of chemical weapons in the Rif War)[32][35]
- ItalyinLibyain 1930[32]
- TheSoviet UnioninXin gian g,Republic of China,during theSoviet Invasion of Xin gian gagainst the36th Division (National Revolutionary Army)in 1934, and also in theXin gian g War (1937)in 1936–37[33][35]
- Italy againstAbyssinia(nowEthiopia) in1935-1936[32]
- TheJapanese EmpireagainstChinain1937–1945[33]
- The US military conducted experiments with chemical weapons like lewisite and mustard gas on Japanese American, Puerto Rican and African Americans in the US military in World War II to see how non-white races would react to being mustard gassed, with Rollin Edwards describing it as "It felt like you were on fire, Guys started screaming and hollering and trying to break out. And then some of the guys fainted. And finally they opened the door and let us out, and the guys were just, they were in bad shape." and "It took all the skin off your hands. Your hands just rotted".[36]
- After WWII, stockpiled mustard gas was dumped by the British in the sea nearPort Elizabeth,South Africa, resulting in many cases of burns among trawler crews[37]
- The United States Government tested effectiveness on US Naval recruits in a laboratory setting at The Great Lakes Naval Base, June 3, 1945[38]
- The 2 December 1943air raid on Baridestroyed an Allied stockpile of mustard gas on theSSJohn Harvey,[39]killing 83 and hospitalizing 628.[40]
- Egypt againstNorth Yemenin 1963–1967[32]
- Iraq againstKurdsin the town ofHalabjaduring theHalabja chemical attackin 1988[33][41]
- Iraq against Iranians in1983–1988[42]
- Possibly in Sudan against insurgents in thecivil war,in 1995 and 1997.[32]
- In theIraq War,abandoned stockpiles of mustard gas shells were destroyed in the open air,[43]and were used against Coalition forces inroadside bombs.[44]
- ByISISforces againstKurdishforces in Iraq in August 2015.[45]
- By ISIS against another rebel group in the town ofMare'in 2015.[46]
- According to Syrian state media, by ISIS against theSyrian Armyduring the battle inDeir ez-Zorin 2016.[47]
The use of toxic gases or other chemicals, including mustard gas, during warfare is known aschemical warfare,and this kind of warfare was prohibited by theGeneva Protocol of 1925,and also by the laterChemical Weapons Convention of 1993.The latter agreement also prohibits the development, production, stockpiling, and sale of such weapons.
In September 2012, a US official stated that the rebel militant groupISISwas manufacturing and using mustard gas in Syria and Iraq, which was allegedly confirmed by the group's head of chemical weapons development, Sleiman Daoud al-Afari, who has since been captured.[48][49]
Development of the first chemotherapy drug[edit]
As early as 1919 it was known that mustard agent was a suppressor ofhematopoiesis.[50]In addition, autopsies performed on 75 soldiers who had died of mustard agent duringWorld War Iwere done by researchers from theUniversity of Pennsylvaniawho reported decreased counts ofwhite blood cells.[40]This led the American Office of Scientific Research and Development (OSRD) to finance the biology and chemistry departments atYale Universityto conduct research on the use of chemical warfare during World War II.[40][51]
As a part of this effort, the group investigatednitrogen mustardas a therapy forHodgkin's lymphomaand other types oflymphomaandleukemia,and this compound was tried out on its first human patient in December 1942. The results of this study were not published until 1946, when they were declassified.[51]In a parallel track, after theair raid on Bariin December 1943, the doctors of the U.S. Army noted that white blood cell counts were reduced in their patients. Some years after World War II was over, the incident in Bari and the work of the Yale University group with nitrogen mustard converged, and this prompted a search for other similarchemical compounds.Due to its use in previous studies, the nitrogen mustard called "HN2" became the first cancerchemotherapydrug,chlormethine(also known as mechlorethamine, mustine) to be used. Chlormethine and other mustard gas molecules are still used to this day as an chemotherapy agent albeit they have largely been replaced with more safe chemotherapy drugs likecisplatinandcarboplatin.[52]
Disposal[edit]
This section needs to beupdated.(February 2022) |
In the United States, storage and incineration of mustard gas and other chemical weapons were carried out by the U.S. Army Chemical Materials Agency.[53]Disposal projects at the two remaining American chemical weapons sites were carried out nearRichmond, Kentucky,andPueblo, Colorado.Although not yet declassified,[specify]toxicology specialists who dealt with the accidental puncturing of World War I gas stockpiles add that Air Force bases in Colorado have been made available to assist veterans of the 2003 American war with Iraq in which many Marines were exposed to gas as caches of up to 25,000 lb (11,000 kg).[citation needed]The United Nations definition of a weapon of mass destruction for mustard gas is 30,000 lb (14,000 kg), typically the Marines and other coalition allies discovered caches of 25,000 pounds (11,000 kg) located across a road from 5,000 pounds (2,300 kg) caches as multiple memoirs attest[citation needed].These were discovered by the assistance of host country allies, or through leaks affecting personnel in an area with a weapon and gas cache called anASP.[citation needed]
New detection techniques are being developed in order to detect the presence of mustard gas and its metabolites. The technology is portable and detects small quantities of the hazardous waste and its oxidized products, which are notorious for harming unsuspecting civilians. Theimmunochromatographicassay would eliminate the need for expensive, time-consuming lab tests and enable easy-to-read tests to protect civilians from sulfur-mustard dumping sites.[54]
In 1946, 10,000 drums of mustard gas (2,800 tonnes) stored at the production facility of Stormont Chemicals inCornwall, Ontario,Canada, were loaded onto 187 boxcars for the 900 miles (1,400 km) journey to be buried at sea on board a 400 foot (120 m) long barge 40 miles (64 km) south ofSable Island,southeast ofHalifax,at a depth of 600 fathoms (1,100 m). The dump location is 42 degrees, 50 minutes north by 60 degrees, 12 minutes west.[55]
A large British stockpile of old mustard agent that had been made and stored since World War I atM. S. Factory, ValleynearRhydymwyninFlintshire,Wales, was destroyed in 1958.[56]
Most of the mustard gas found in Germany afterWorld War IIwas dumped into theBaltic Sea.Between 1966 and 2002, fishermen have found about 700 chemical weapons in the region ofBornholm,most of which contain mustard gas. One of the more frequently dumped weapons was "Sprühbüchse 37" (SprüBü37, Spray Can 37, 1937 being the year of its fielding with the German Army). These weapons contain mustard gas mixed with athickener,which gives it a tar-like viscosity. When the content of the SprüBü37 comes in contact with water, only the mustard gas in the outer layers of the lumps of viscous mustardhydrolyzes,leaving behind amber-colored residues that still contain most of the active mustard gas. On mechanically breaking these lumps (e.g., with the drag board of a fishing net or by the human hand) the enclosed mustard gas is still as active as it had been at the time the weapon was dumped. These lumps, when washed ashore, can be mistaken for amber, which can lead to severe health problems.Artillery shellscontaining mustard gas and other toxic ammunition from World War I (as well as conventional explosives) can still be found in France and Belgium. These were formerly disposed of by explosion undersea, but since the current environmental regulations prohibit this, theFrench governmentis building an automated factory to dispose of the accumulation of chemical shells.
In 1972, theU.S. Congressbanned the practice of disposing of chemical weapons into the ocean by the United States. 29,000 tons of nerve and mustard agents had already been dumped into the ocean off the United States by theU.S. Army.According to a report created in 1998 by William Brankowitz, a deputy project manager in theU.S. Army Chemical Materials Agency,the army created at least 26 chemical weapons dumping sites in the ocean offshore from at least 11 states on both theEast Coastand theWest Coast(inOperation CHASE,Operation Geranium,etc.). In addition, due to poor recordkeeping, about one-half of the sites have only their rough locations known.[57]
In June 1997, India declared its stock of chemical weapons of 1,044 tonnes (1,151 short tons) of mustard gas.[58][59]By the end of 2006, India had destroyed more than 75 percent of its chemical weapons/material stockpile and was granted extension for destroying the remaining stocks by April 2009 and was expected to achieve 100 percent destruction within that time frame.[58]India informed the United Nations in May 2009 that it had destroyed its stockpile of chemical weapons in compliance with the international Chemical Weapons Convention. With this India has become the third country after South Korea and Albania to do so.[60][61]This was cross-checked by inspectors of the United Nations.
Producing or stockpiling mustard gas is prohibited by theChemical Weapons Convention.When the convention entered force in 1997, the parties declared worldwide stockpiles of 17,440 tonnes of mustard gas. As of December 2015, 86% of these stockpiles had been destroyed.[62]
A significant portion of the United States' mustard agentstockpilewas stored at the Edgewood Area ofAberdeen Proving GroundinMaryland.Approximately 1,621 tons of mustard agents were stored in one-ton containers on the base under heavy guard. A chemical neutralization plant was built on the proving ground and neutralized the last of this stockpile in February 2005. This stockpile had priority because of the potential for quick reduction of risk to the community. The nearest schools were fitted with overpressurization machinery to protect the students and faculty in the event of a catastrophic explosion and fire at the site. These projects, as well as planning, equipment, and training assistance, were provided to the surrounding community as a part of the Chemical Stockpile Emergency Preparedness Program (CSEPP), a joint program of the Army and theFederal Emergency Management Agency(FEMA).[63]Unexploded shells containing mustard gases and other chemical agents are still present in several test ranges in proximity to schools in the Edgewood area, but the smaller amounts of poison gas (4 to 14 pounds (1.8 to 6.4 kg)) present considerably lower risks. These remnants are being detected and excavated systematically for disposal. The U.S. Army Chemical Materials Agency oversaw disposal of several other chemical weapons stockpiles located across the United States in compliance with international chemical weapons treaties. These include the complete incineration of the chemical weapons stockpiled inAlabama,Arkansas,Indiana,andOregon.Earlier, this agency had also completed destruction of the chemical weapons stockpile located onJohnston Atolllocated south ofHawaiiin thePacific Ocean.[64]The largest mustard agent stockpile, at approximately 6,200short tons,was stored at theDeseret Chemical Depotin northernUtah.The incineration of this stockpile began in 2006. In May 2011, the last of the mustard agents in the stockpile were incinerated at the Deseret Chemical Depot, and the last artillery shells containing mustard gas were incinerated in January 2012.
In 2008, many emptyaerial bombsthat contained mustard gas were found in an excavation at theMarrangaroo Army Basejust west of Sydney, Australia.[65][66]In 2009, a mining survey nearChinchilla, Queensland,uncovered 144 105-millimeterhowitzershells, some containing "Mustard H", that had been buried by the U.S. Army during World War II.[66][67]
In 2014, a collection of 200 bombs was found near theFlemishvillages ofPassendaleandMoorslede.The majority of the bombs were filled with mustard agents. The bombs were left over from the German army and were meant to be used in theBattle of Passchendaelein World War I. It was the largest collection of chemical weapons ever found in Belgium.[68]
A large amount of chemical weapons, including mustard gas, was found in a neighborhood ofWashington, D.C.The cleanup was completed in 2021.[69]
Post-war accidental exposure[edit]
In 2002, an archaeologist at the Presidio Trust archaeology lab in San Francisco was exposed to mustard gas, which had been dug up at thePresidio of San Francisco,a former military base.[70]
In 2010, a clamming boat pulled up some oldartilleryshells of World War I from theAtlantic Oceansouth ofLong Island, New York.Multiple fishermen suffered from blistering and respiratory irritation severe enough to require hospitalization.[71]
WWII-era tests on men[edit]

From 1943 to 1944, mustard agent experiments were performed on Australian service volunteers in tropicalQueensland, Australia,byBritish Armyand American experimenters, resulting in some severe injuries. One test site, theBrook Islands National Park,was chosen to simulate Pacific islands held by theImperial Japanese Army.[72][73]
The United States tested sulfur mustards and other chemical agents includingnitrogen mustardsandlewisiteon up to 60,000 servicemen during and after WWII. The experiments were classified secret and as withAgent Orange,claims for medical care and compensation were routinely denied, even after the WWII-era tests were declassified in 1993. TheDepartment of Veterans Affairsstated that it would contact 4,000 surviving test subjects but failed to do so, eventually only contacting 600. Skin cancer, severe eczema, leukemia, and chronic breathing problems plagued the test subjects, some of whom were as young as 19 at the time of the tests, until their deaths, but even those who had previously filed claims with the VA went without compensation.[74]

African American servicemenwere tested alongside white men in separate trials to determine whether their skin color would afford them a degree of immunity to the agents, andNiseiservicemen, some of whom had joined after their release fromJapanese American Internment Campswere tested to determine susceptibility ofJapanese military personnelto these agents. These tests also includedPuerto Ricansubjects.[75]
Detection in biological fluids[edit]
Concentrations of thiodiglycol in urine have been used to confirm a diagnosis of chemical poisoning in hospitalized victims. The presence in urine of 1,1'-sulfonylbismethylthioethane (SBMTE), a conjugation product with glutathione, is considered a more specific marker, since this metabolite is not found in specimens from unexposed persons. In one case, intact mustard gas was detected in postmortem fluids and tissues of a man who died one week post-exposure.[76]
See also[edit]
- Bis(chloromethyl) ether
- Chlorine gas
- Keen as Mustard
- Phosgene oxime
- Poison gas in World War I
- Rawalpindi experiments
- Selenium mustard
References[edit]
Notes
- ^abcdeFM 3–8 Chemical Reference handbook, US Army, 1967
- ^abcdeMustard agents: description, physical and chemical properties, mechanism of action, symptoms, antidotes and methods of treatment.Organisation for the Prohibition of Chemical Weapons. Accessed June 8, 2010.
- ^"Pubchem".
- ^ab"Mustard Gas"(PDF).ChemMatters.American Chemical Society.
- ^"What is a Chemical Weapon?".OPCW.Retrieved2023-09-15.
- ^Salouti, Ramin; Ghazavi, Roghayeh; Rajabi, Sattar; Zare, Mohammad; Talebnejad, Mohammadreza; Abtahi, Mohammad Bagher; Parvizi, Maryam; Madani, Sedigheh; Asadi-Amoli, Fahimeh; Mirsharif, Ensieh-Sadat; Gharebaghi, Reza (2020)."Sulfur Mustard and Immunology; Trends of 20 Years Research in the Web of Science Core Collection: A Scientometric Review".Iranian Journal of Public Health.49(7): 1202–1210.doi:10.18502/ijph.v49i7.3573.ISSN2251-6085.PMC7548481.PMID33083286.
- ^Watson, A. P.; Griffin, G. D. (1992)."Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program".Environmental Health Perspectives.98:259–280.doi:10.1289/ehp.9298259.ISSN0091-6765.PMC1519623.PMID1486858.
- ^Smith, Susan L. (27 February 2017)."War! What is it good for? Mustard gas medicine".CMAJ.189(8): E321–E322.doi:10.1503/cmaj.161032.PMC5325736.PMID28246228.
- ^Ghasemi, Hassan; Javadi, Mohammad Ali; Ardestani, Sussan K.; Mahmoudi, Mahmoud; Pourfarzam, Shahryar; Mahdavi, Mohammad Reza Vaez; Yarmohammadi, Mohammad Ebrahim; Baradaran-Rafii, Alireza; Jadidi, Khosro; Shariatpanahi, Shamsa; Rastin, Maryam (2020)."Alteration in inflammatory mediators in seriously eye-injured war veterans, long-term after sulfur mustard exposure".International Immunopharmacology.80:105897.doi:10.1016/j.intimp.2019.105897.ISSN1878-1705.PMID31685435.S2CID207899509.
- ^Ghazanfari, Tooba; Ghasemi, Hassan; Yaraee, Roya; Mahmoudi, Mahmoud; Javadi, Mohammad Ali; Soroush, Mohammad Reza; Faghihzadeh, Soghrat; Majd, Ali Mohammad Mohseni; Shakeri, Raheleh; Babaei, Mahmoud; Heidary, Fatemeh (2019)."Tear and serum interleukin-8 and serum CX3CL1, CCL2 and CCL5 in sulfur mustard eye-exposed patients".International Immunopharmacology.77:105844.doi:10.1016/j.intimp.2019.105844.ISSN1878-1705.PMID31669888.S2CID204967476.
- ^Heidary, Fatemeh; Gharebaghi, Reza; Ghasemi, Hassan; Mahdavi, Mohammad Reza Vaez; Ghaffarpour, Sara; Naghizadeh, Mohammad Mehdi; Ghazanfari, Tooba (2019)."Angiogenesis modulatory factors in subjects with chronic ocular complications of Sulfur Mustard exposure: A case-control study".International Immunopharmacology.76:105843.doi:10.1016/j.intimp.2019.105843.ISSN1878-1705.PMID31629219.S2CID204799405.
- ^Heidary, Fatemeh; Ardestani, Sussan K.; Ghasemi, Hassan; Javadi, Mohammad Ali; Mahmoudi, Mahmoud; Yaraee, Roya; Shams, Jalaledin; Falahi, Faramarz; Sedighi Moghadam, Mohamad Reza; Shariatpanahi, Shamsa; Shakeri, Raheleh (2019)."Alteration in serum levels of ICAM-1 and P-, E- and L-selectins in seriously eye-injured long-term following sulfur-mustard exposure".International Immunopharmacology.76:105820.doi:10.1016/j.intimp.2019.105820.ISSN1878-1705.PMID31480003.S2CID201831881.
- ^Safarinejad, M. R.; Moosavi, S. A.; Montazeri, B (2001)."Ocular injuries caused by mustard gas: diagnosis, treatment, and medical defense".Military Medicine.166(1): 67–70.doi:10.1093/milmed/166.1.67.PMID11197102.
- ^Vesicants.brooksidepress.org
- ^Effects of mustard gas, WW1|Gas Warfare Medical Aspects|World War II Resource Centre.Vlib.us (2004-08-23). Retrieved on 2011-05-29.
- ^Najafi, Ali; Masoudi-Nejad, Ali; Imani Fooladi, Abbas Ali; Ghanei, Mostafa; Nourani, Mohamad Reza (2014)."Microarray gene expression analysis of the human airway in patients exposed to sulfur mustard".Journal of Receptors and Signal Transduction.34(4): 283–9.doi:10.3109/10799893.2014.896379.PMID24823320.S2CID41665583.
- ^Ghasemi, Hassan; Javadi, Mohammad Ali; Ardestani, Sussan K.; Mahmoudi, Mahmoud; Pourfarzam, Shahryar; Mahdavi, Mohammad Reza Vaez; Yarmohammadi, Mohammad Ebrahim; Baradaran-Rafii, Alireza; Jadidi, Khosro; Shariatpanahi, Shamsa; Rastin, Maryam (2020)."Alteration in inflammatory mediators in seriously eye-injured war veterans, long-term after sulfur mustard exposure".International Immunopharmacology.80:105897.doi:10.1016/j.intimp.2019.105897.PMID31685435.S2CID207899509.
- ^Geraci, Matthew J. (2008)."Mustard gas: imminent danger or eminent threat?".The Annals of Pharmacotherapy.42(2): 237–246.doi:10.1345/aph.1K445.ISSN1542-6270.PMID18212254.S2CID207263000.
- ^Dabrowska, Milena I.; Becks, Lauren L.; Lelli, Joseph L. Jr.; Levee, Minette G.; Hinshaw, Daniel B. (1996). "Sulfur Mustard Induces Apoptosis and Necrosis in Endothelial Cells".Toxicology and Applied Pharmacology.141(2): 568–83.Bibcode:1996ToxAP.141..568D.doi:10.1006/taap.1996.0324.PMID8975783.
- ^Van Bergen, Leo (2009).Before My Helpless Sight: Suffering, Dying and Military Medicine on the Western Front, 1914–1918.Ashgate Publishing, Ltd. p. 184.ISBN978-0-7546-5853-5.
- ^Stewart, Charles D. (2006).Weapons of mass casualties and terrorism response handbook.Boston: Jones and Bartlett. p. 47.ISBN0-7637-2425-4.
- ^"Chemical Weapons Production and Storage".Federation of American Scientists. Archived fromthe originalon August 11, 2014.
- ^The Emergency Response Safety and Health Database: Mustard-Lewisite Mixture (HL).National Institute for Occupational Safety and Health. Accessed March 19, 2009.
- ^By Any Other Name: Origins of Mustard GasArchived2014-02-01 at theWayback Machine.Itech.dickinson.edu (2008-04-25). Retrieved on 2011-05-29.
- ^F. Guthrie (1860)."XIII.—On some derivatives from the olefines".Q. J. Chem. Soc.12(1): 109–126.doi:10.1039/QJ8601200109.
- ^Duchovic, Ronald J.; Vilensky, Joel A. (2007). "Mustard Gas: Its Pre-World War I History".J. Chem. Educ.84(6): 944.Bibcode:2007JChEd..84..944D.doi:10.1021/ed084p944.
- ^Fries, Amos A. (Amos Alfred); West, Clarence J. (Clarence Jay) (1921).Chemical warfare.University of California Libraries. New York [etc.] McGraw-Hill Book Company, inc. p. 176.
(...) on the night of July 12, 1917 (...)
- ^Fries, Amos A. (Amos Alfred); West, Clarence J. (Clarence Jay) (1921).Chemical warfare.University of California Libraries. New York [etc.] McGraw-Hill Book Company, inc. p. 150.
(...) 'Ypres,' a name used by the French, because the compound was first used at Ypres (...)
- ^David Large (ed.).The Port of Bristol, 1848-1884.
- ^"Photographic Archive of Avonmouth Bristol BS11".BristolPast.co.uk. Archived fromthe originalon July 3, 2011.Retrieved12 May2014.
- ^Fischer, Karin (June 2004). Schattkowsky, Martina (ed.).Steinkopf, Georg Wilhelm, in: Sächsische Biografie(in German) (Online ed.). Institut für Sächsische Geschichte und Volkskunde.Retrieved2010-12-28.
- ^abcdefBlister Agent: Mustard gas (H, HD, HS)ArchivedJuly 24, 2007, at theWayback Machine,CBWinfo
- ^abcdPearson, Graham S."Uses of CW since the First World War".Federation of American Scientitst. Archived fromthe originalon August 22, 2010.Retrieved2010-06-28.
- ^Townshend, Charles(1986). "Civilisation and" Frightfulness ": Air Control in the Middle East Between the Wars". In Chris Wrigley (ed.).Warfare, diplomacy and politics: essays in honour of A.J.P. Taylor.Hamilton. p. 148.ISBN978-0-241-11789-7.
- ^abDaniel Feakes (2003)."Global society and biological and chemical weapons"(PDF).In Mary Kaldor; Helmut Anheier; Marlies Glasius (eds.).Global Civil Society Yearbook 2003.Oxford University Press. pp. 87–117.ISBN0-19-926655-7.Archived fromthe original(PDF)on 2007-07-11.
- ^Dickerson, Caitlin (22 June 2015)."Secret World War II Chemical Experiments Tested Troops By Race".NPR.
- ^"NEWSLETTER - JUNE 1992 NEWSLETTER - Johannesburg - South African Military History Society - Title page".Samilitaryhistory.org.Retrieved2013-08-23.
- ^"The Tox Lab: When U Chicago Was in the Chemical Weapons" Business "| Newcity".2013-09-23.Retrieved2021-07-02.
- ^K. Coleman (23 May 2005).A History of Chemical Warfare.Palgrave Macmillan UK. pp. 74–.ISBN978-0-230-50183-6.
- ^abcFaguet, Guy B. (2005).The War on Cancer.Springer. p. 71.ISBN1-4020-3618-3.
- ^Lyon, Alistair (2008-07-09)."Iran's Chemical Ali survivors still bear scars".Reuters.Retrieved2008-11-17.
- ^Benschop, Hendrik P.; van der Schans, Govert P.; Noort, Daan; Fidder, Alex; Mars-Groenendijk, Roos H.; de Jong, Leo P.A. (1 July 1997)."Verification of Exposure to Sulfur Mustard in Two Casualties of the Iran-Iraq Conflict".Journal of Analytical Toxicology.21(4): 249–251.doi:10.1093/jat/21.4.249.PMID9248939.
- ^"More Than 600 Reported Chemical Exposure in Iraq, Pentagon Acknowledges".The New York Times.6 Nov 2014.
- ^"Veterans Hurt by Chemical Weapons in Iraq Get Apology".The New York Times.25 Mar 2015.
- ^Deutsch, Anthony (15 February 2016)."Samples confirm Islamic State used mustard gas in Iraq - diplomat".Reuters.Retrieved15 February2016.
- ^Deutsch, Anthony (2015-11-06)."Chemical weapons used by fighters in Syria—sources".Reuters.Retrieved2017-06-30.
- ^"Syria war: IS 'used mustard gas' on Assad troops".BBC News.2016-04-05.Retrieved2017-06-30.
- ^Paul Blake (11 September 2015)."US official: 'IS making and using chemical weapons in Iraq and Syria'".BBC.Retrieved16 September2015.
- ^Lizzie Dearden (11 September 2015)."Isis 'manufacturing and using chemical weapons' in Iraq and Syria, US official claims".The Independent.Archivedfrom the original on 2022-06-18.Retrieved16 September2015.
- ^Krumbhaar EB (1919)."Rôle of the blood and the bone marrow in certain forms of gas poisoning: I. peripheral blood changes and their significance".JAMA.72:39–41.doi:10.1001/jama.1919.26110010018009f.
- ^abGilman A (May 1963). "The initial clinical trial of nitrogen mustard".Am. J. Surg.105(5): 574–8.doi:10.1016/0002-9610(63)90232-0.PMID13947966.
- ^Scott, Lesley J. (2017-06-01)."Chlormethine 160 mcg/g gel in mycosis fungoides-type cutaneous T-cell lymphoma: a profile of its use in the EU".Drugs & Therapy Perspectives.33(6): 249–253.doi:10.1007/s40267-017-0409-7.ISSN1179-1977.S2CID256367068.
- ^The U.S. Army's Chemical Materials Agency (CMA)ArchivedOctober 15, 2004, at theWayback Machine.cma.army.mil. Retrieved on November 11, 2011.
- ^Sathe, Manisha; Srivastava, Shruti; Merwyn, S.; Agarwal, G. S.; Kaushik, M. P. (24 July 2014)."Competitive immunochromatographic assay for the detection of thiodiglycol sulfoxide, a degradation product of sulfur mustard".The Analyst.139(20): 5118–26.Bibcode:2014Ana...139.5118S.doi:10.1039/C4AN00720D.PMID25121638.
- ^"Hill 70 & Cornwall's Deadly Mustard Gas Plant".Cornwall Community Museum.Stormont, Dundas and Glengarry Historical Society. 18 September 2016.Retrieved23 December2016.
- ^"Valley Factory, Rhydymwyn".24 July 2010.
- ^Bull, John (30 October 2005)."The Deadliness Below".Daily Press Virginia. Archived fromthe originalon 2012-07-23.Retrieved2013-01-28.
- ^ab"India to destroy chemical weapons stockpile by 2009".Dominican Today. Archived fromthe originalon 7 September 2013.Retrieved30 April2013.
- ^Amy Smithson; Frank Gaffney Jr."India declares its stock of chemical weapons".Archived fromthe originalon 6 November 2012.Retrieved30 April2013.
- ^"Zee News – India destroys its chemical weapons stockpile".Zeenews.india. 14 May 2009.Retrieved30 April2013.
- ^"India destroys its chemical weapons stockpile - Yahoo! India News".Archived fromthe originalon 21 May 2009.Retrieved20 May2009.
- ^Organisation for the Prohibition of Chemical Weapons (30 November 2016)."Annex 3".Report of the OPCW on the Implementation of the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction in 2015 (Report). p. 42.Retrieved8 March2017.
- ^"CSEPP Background Information".US Federal Emergency Management Agency (FEMA). 2 May 2006. Archived fromthe originalon 27 May 2006.
- ^"Milestones in U.S. Chemical Weapons Storage and Destruction, fact sheet, US Chemical Materials Agency".Archived fromthe originalon 15 September 2012.Retrieved15 January2012.
- ^Ashworth L (7 August 2008)."Base's phantom war reveals its secrets".Fairfax Digital. Archived fromthe originalon 5 December 2008.
- ^abChemical Warfare in Australia.Mustardgas.org. Retrieved on 29 May 2011.
- ^Cumming, Stuart (11 November 2009)."Weapons await UN inspection".Toowoomba Chronicle.
- ^"Farmer discovers 200 bombs (Dutch)".5 March 2014.
- ^"Cleanup Complete At WWI Chemical Weapons Dump In D.C.'s Spring Valley".DCist.Archivedfrom the original on 2022-02-07.Retrieved2022-02-07.
- ^Sullivan, Kathleen (2002-10-22)."Vial found in Presidio may be mustard gas / Army experts expected to identify substance".sfgate.
- ^Wickett, Shana; Beth Daley (2010-06-08)."Fishing crewman exposed to mustard gas from shell".The Boston Globe.Archived fromthe originalon June 9, 2010.
- ^Goodwin, Bridget (1998).Keen as mustard: Britain's horrific chemical warfare experiments in Australia.St. Lucia: University of Queensland Press.ISBN978-0-7022-2941-1.
- ^Brook Island Trials of Mustard Gas during WW2.Home.st.net.au. Retrieved on 2011-05-29.
- ^Dickerson, Caitlin (2015-06-23)."The VA's Broken Promise To Thousands Of Vets Exposed To Mustard Gas".NPR.Retrieved2019-05-03.
... the Department of Veterans Affairs made two promises: to locate about 4,000 men who were used in the most extreme tests, and to compensate those who had permanent injuries.
- ^Dickerson, Caitlin (2015-06-22)."Secret World War II Chemical Experiments Tested Troops By Race".NPR.Retrieved2019-05-03.
And it wasn't just African-Americans. Japanese-Americans were used [...] so scientists could explore how mustard gas and other chemicals might affect Japanese troops. Puerto Rican soldiers were also singled out.
- ^R. Baselt,Disposition of Toxic Drugs and Chemicals in Man,10th edition, Biomedical Publications, Seal Beach, CA, 2014, pp. 1892–1894.
Further reading[edit]
- Cook, Tim. "‘Against God-Inspired Conscience’: The Perception of Gas Warfare as a Weapon of Mass Destruction, 1915–1939."War & Society18.1 (2000): 47-69.
- Dorsey, M. Girard.Holding Their Breath: How the Allies Confronted the Threat of Chemical Warfare in World War II(Cornell UP, 2023)online.
- Duchovic, Ronald J., and Joel A. Vilensky. "Mustard gas: its pre-World War I history."Journal of chemical education84.6 (2007): 944.online
- Feister, Alan J.Medical defense against mustard gas: toxic mechanisms and pharmacological implications(1991).online
- Fitzgerald, Gerard J. "Chemical warfare and medical response during World War I."American journal of public health98.4 (2008): 611-625.online
- *Freemantle, M. (2012).Gas! GAS! Quick, boys! How Chemistry Changed the First World War.The History Press.ISBN978-0-7524-6601-9.
- Geraci, Matthew J. "Mustard gas: imminent danger or eminent threat?."Annals of Pharmacotherapy42.2 (2008): 237-246.online
- Ghabili, Kamyar, et al. "Mustard gas toxicity: the acute and chronic pathological effects."Journal of applied toxicology30.7 (2010): 627-643.online
- Jones, Edgar. "Terror weapons: The British experience of gas and its treatment in the First World War."War in History21.3 (2014): 355-375.online
- MacPherson, W. G.; Herringham, W. P.; Elliott, T. R.; Balfour, A. (1923).Medical Services: Diseases of the War: Including the Medical Aspects of Aviation and Gas Warfare and Gas Poisoning in Tanks and Mines.History of the Great War Based on Official Documents by Direction of the Historical Section of the Committee of Imperial Defence. Vol. II. London: HMSO.OCLC769752656.Retrieved19 October2014.
- Padley, Anthony Paul. "Gas: the greatest terror of the Great War."Anaesthesia and intensive care44.1_suppl (2016): 24-30.online
- Rall, David P., and Constance M. Pechura, eds.Veterans at risk: The health effects of mustard gas and lewisite(1993).online
- Richter, Donald (1994).Chemical Soldiers.Leo Cooper.ISBN0850523885.
- Schummer, Joachim. "Ethics of chemical weapons research: Poison gas in World War One."Ethics of Chemistry: From Poison Gas to Climate Engineering(2021) pp. 55-83.online
- Smith, Susan I.Toxic Exposures: Mustard Gas and the Health Consequences of World War II in the United States(Rutgers University Press, 2017)online book review
- Wattana, Monica, and Tareg Bey. "Mustard gas or sulfur mustard: an old chemical agent as a new terrorist threat."Prehospital and disaster medicine24.1 (2009): 19-29.online
External links[edit]
- Mustard gas (Sulphur Mustard) (IARC Summary & Evaluation, Supplement7, 1987).Inchem.org (1998-02-09). Retrieved on 2011-05-29.
- Institute of Medicine (1993)."History and Analysis of Mustard Agent and Lewisite Research Programs in the United States".Veterans at Risk: The Health Effects of Mustard Gas and Lewisite.National Academies Press.ISBN978-0-309-04832-3.
- "CDC - Facts About Sulfur Mustard".cdc.gov.Archived fromthe originalon 2006-08-09.
- "NATO Presses New Libyan Leaders to Eliminate Mustard Agent - Global Security Newswire - NTI".NTI: Nuclear Threat Initiative.Archived fromthe originalon 2016-01-02.Retrieved2015-11-06.
- Textbook of Military Medicine – Intensive overview of mustard gasIncludes many references to scientific literature
- Detailed information on physical effects and suggested treatments
- Iyriboz Y (2004)."A Recent Exposure to Mustard Gas in the United States: Clinical Findings of a Cohort (n = 247) 6 Years After Exposure".MedGenMed.6(4): 4.PMC1480580.PMID15775831.Shows photographs taken in 1996 showing people with mustard gas burns.
- An overview of the sulfur and nitrogen mustard agents(Caution: contains graphic images)
- Questions and Answers for Mustard Gas
- UMDNJ-Rutgers University CounterACT Research Center of ExcellenceA research center studying mustard gas, includessearchable reference librarywith many early references on mustard gas.
- Clayton, William; Howard, Alfred John; Thomson, David (25 May 1946)."Treatment of Mustard Gas Burns".British Medical Journal.1(4455): 797–799.doi:10.1136/bmj.1.4455.797.PMC2058956.PMID20786722.
- Nightmare in Bari
- surgical treatment of mustard gas burns
- UK Ministry of Defence Report on disposal of weapons at sea and incidents arising
- Rhydymwyn Valley History Society
- The advent of mustard gas in 1917, Simon Jones
- Measures to protect against mustard gas, 1917-1918, Simon Jones

