n-Butyllithium
| |||
 Close-up of the delocalized bonds between butyl and lithium
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
butyllithium, tetra-μ3-butyl-tetralithium
| |||
| Other names
NBL, BuLi,
1-lithiobutane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.363 | ||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H9Li | |||
| Molar mass | 64.06g·mol−1 | ||
| Appearance | colorless liquid unstable usually obtained as solution | ||
| Density | 0.68 g/cm3,solvent defined | ||
| Melting point | −76 °C (−105 °F; 197 K) (<273 K) | ||
| Boiling point | 80 C | ||
| Exothermic decomposition | |||
| Solubility | Ethers such asTHF,hydrocarbons | ||
| Acidity(pKa) | 50 (of the conjugate acid)[1] | ||
| Structure | |||
| tetrameric in solution | |||
| 0D | |||
| Hazards | |||
| Occupational safety and health(OHS/OSH): | |||
Main hazards
|
Pyrophoric (spontaneously combusts in air), decomposes to corrosiveLiOH | ||
| NFPA 704(fire diamond) | |||
| Related compounds | |||
Relatedorganolithium
reagents |
sec-butyllithium tert-butyllithium hexyllithium methyllithium | ||
Related compounds
|
lithium hydroxide | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||

n-ButyllithiumC4H9Li (abbreviatedn-BuLi) is anorganolithium reagent.It is widely used as apolymerizationinitiator in the production ofelastomerssuch aspolybutadieneorstyrene-butadiene-styrene (SBS).Also, it is broadly employed as a strongbase(superbase) in thesynthesis of organic compoundsas in the pharmaceutical industry.
Butyllithium is commercially available as solutions (15%, 25%, 1.5M,2 M, 2.5 M, 10 M, etc.) inalkanessuch aspentane,hexanes,andheptanes.Solutions indiethyl etherandTHFcan be prepared, but are not stable enough for storage. Annual worldwide production and consumption of butyllithium and other organolithium compounds is estimated at 2000 to 3000 tonnes.[2]
Although butyllithium is colorless,n-butyllithium is usually encountered as a pale yellow solution in alkanes. Such solutions are stable indefinitely if properly stored,[3]but in practice, they degrade upon aging. Fine white precipitate (lithium hydride) is deposited and the color changes to orange.[3][4]
Structure and bonding
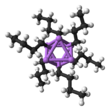
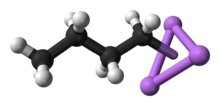
[edit]n-BuLi exists as a cluster both in the solid state and in a solution. The tendency to aggregate is common for organolithium compounds. The aggregates are held together by delocalized covalent bonds between lithium and the terminal carbon of the butyl chain.[5]In the case ofn-BuLi, the clusters are tetrameric (in ether) or hexameric (incyclohexane). The cluster is a distortedcubane-type clusterwith Li andCH2R groups at alternating vertices. An equivalent description describes the tetramer as a Li4tetrahedroninterpenetrated with a tetrahedron [CH2R]4.Bonding within the cluster is related to that used to describe diborane, but more complex since eight atoms are involved. Reflecting its electron-rich character,n-butyllithium is highly reactive towardLewis acids.
Due to the large difference between theelectronegativitiesofcarbon(2.55) andlithium(0.98), the C−Li bond is highly polarized. The charge separation has been estimated to be 55–95%. For practical purposes,n-BuLi can often be considered to react as the butylanion,n-Bu−,and a lithiumcation,Li+.
Preparation
[edit]The standard preparation forn-BuLi is reaction of1-bromobutaneor1-chlorobutanewith Li metal:[3]
- 2 Li + C4H9X → C4H9Li + LiX(X = Cl, Br)
If the lithium used for this reaction contains 1–3%sodium,the reaction proceeds more quickly than if pure lithium is used. Solvents used for this preparation includebenzene,cyclohexane, and diethyl ether. When BuBr is the precursor, the product is a homogeneous solution, consisting of a mixed cluster containing both LiBr and BuLi, together with a small amount ofoctane.BuLi forms a weaker complex with LiCl, so that the reaction of BuCl with Li produces a precipitate ofLiCl.
Solutions of butyllithium, which are susceptible to degradation by air, are standardized bytitration.A popular weak acid isbiphenyl-4-methanol, which gives a deeply colored dilithio derivative at the end point.[6]
Applications
[edit]Butyllithium is principally valued as an initiator for the anionicpolymerizationofdienes,such asbutadiene.[7]The reaction is called "carbolithiation":
- C4H9Li + CH2=CH−CH=CH2→ C4H9−CH2−CH=CH−CH2Li
Isoprenecan be polymerized stereospecifically in this way. Also of commercial importance is the use of butyllithium for the production ofstyrene-butadienepolymers. Evenethylenewill insert into BuLi.[8]
Reactions
[edit]Butyllithium is a strong base (pKb≈ -36), but it is also a powerfulnucleophileandreductant,depending on the other reactants. Furthermore, in addition to being a strong nucleophile,n-BuLi binds to aprotic Lewis bases, such as ethers and tertiaryamines,which partially disaggregate the clusters by binding to the lithium centers. Its use as a strongbaseis referred to asmetalation.Reactions are typically conducted intetrahydrofurananddiethyl ether,which are good solvents for the resulting organolithium derivatives (see below).
Metalation
[edit]One of the most useful chemical properties ofn-BuLi is its ability to deprotonate a wide range of weakBrønsted acids.t-Butyllithium ands-butyllithium are more basic.n-BuLi can deprotonate (that is, metalate) many types of C−H bonds, especially where theconjugate baseis stabilized by electrondelocalizationor one or more heteroatoms (non-carbon atoms). Examples include acetylenes (H−CC−R), methyl sulfides (H−CH2SR), thioacetals (H−CH(SR)2,e.g.dithiane), methylphosphines (H−CH2PR2),furans,thiophenesandferrocene(Fe(H−C5H4)(C5H5)).[9]In addition to these, it will also deprotonate all more acidic compounds such as alcohols, amines,enolizablecarbonyl compounds, and any overtly acidic compounds, to produce alkoxides, amides, enolates and other salts of lithium, respectively. The stability andvolatilityof thebutaneresulting from suchdeprotonationreactions is convenient, but can also be a problem for large-scale reactions because of the volume of a flammable gas produced.
- LiC4H9+ RH → C4H10+ RLi
The kinetic basicity ofn-BuLi is affected by the solvent or cosolvent. Ligands that complex Li+such astetrahydrofuran(THF),tetramethylethylenediamine(TMEDA),hexamethylphosphoramide(HMPA), and 1,4-diazabicyclo[2.2.2]octane (DABCO) further polarize the Li−C bond and accelerate the metalation. Such additives can also aid in the isolation of the lithiated product, a famous example of which is dilithioferrocene.
- Fe(C5H5)2+ 2 LiC4H9+ 2 TMEDA → 2 C4H10+ Fe(C5H4Li)2(TMEDA)2
Schlosser's baseis asuperbaseproduced by treating butyllithium withpotassiumt-butoxide.It is kinetically more reactive than butyllithium and is often used to accomplish difficultmetalations.While somen-butylpotassium is present and is a stronger base thann-BuLi, the reactivity of the mixture is not exactly the same as isolatedn-butylpotassium.[10]
An example of the use ofn-butyllithium as a base is the addition of an amine to methyl carbonate to form a methylcarbamate,wheren-butyllithium serves to deprotonate the amine:
- n-BuLi + R2NH + (MeO)2CO → R2NCO2Me + LiOMe + BuH
Halogen–lithium exchange
[edit]Butyllithium reacts with some organic bromides and iodides in an exchange reaction to form the corresponding organolithium derivative. The reaction usually fails with organic chlorides and fluorides:
- C4H9Li + RX → C4H9X + RLi(X = Br, I)
Thislithium–halogen exchangereaction is useful for preparation of several types of RLi compounds, particularlyaryllithium and somevinyllithium reagents. The utility of this method is significantly limited, however, by the presence in the reaction mixture ofn-BuBr orn-BuI, which can react with the RLi reagent formed, and by competingdehydrohalogenationreactions, in whichn-BuLi serves as a base:
- 2 C4H9Br + RLi → 2 C4H9R + LiBr
- 2 C4H9Li + R′CH=CHBr → 2 C4H10+ R′C≡CLi + LiBr
These side reaction are significantly less important for RI than for RBr, since the iodine–lithium exchange is several orders of magnitude faster than the bromine–lithium exchange. For these reasons, aryl, vinyl and primary alkyl iodides are the preferred substrates, andt-BuLirather thann-BuLi is usually used, since the formedt-BuI is immediately destroyed by thet-BuLi in a dehydrohalogenation reaction (thus requiring two equivalents oft-BuLi). Alternatively, vinyl lithium reagents can be generated by direct reaction of the vinyl halide (e.g. cyclohexenyl chloride) with lithium or by tin–lithium exchange (see next section).[3]
Transmetalations
[edit]A related family of reactions are thetransmetalations,wherein two organometallic compounds exchange their metals. Many examples of such reactions involve lithium exchange withtin:
- C4H9Li + Me3SnAr → C4H9SnMe3+ LiAr(where Ar is aryl and Me is methyl)
The tin–lithium exchange reactions have one major advantage over the halogen–lithium exchanges for the preparation of organolithium reagents, in that the product tin compounds (C4H9SnMe3in the example above) are much less reactive towards lithium reagents than are the halide products of the corresponding halogen–lithium exchanges (C4H9Br or C4H9Cl). Othermetalsandmetalloidswhich undergo such exchange reactions are organic compounds ofmercury,selenium,andtellurium.
Carbonyl additions
[edit]Organolithium reagents, includingn-BuLi are used in synthesis of specificaldehydesandketones.One such synthetic pathway is the reaction of an organolithium reagent with disubstitutedamides:
- R1Li + R2CONMe2→ LiNMe2+ R2C(O)R1
Degradation of THF
[edit]THF is deprotonated by butyllithium, especially in the presence ofTMEDA,by loss of one of four protons adjacent to oxygen. This process, which consumes butyllithium to generate butane, induces a ring opening to give enolate ofacetaldehydeandethylene.[11]Therefore, reactions of BuLi in THF are typically conducted at low temperatures, such as –78 °C, as is conveniently produced by afreezing bathofdry iceand acetone. Higher temperatures (−25 °C or even −15 °C) are also used.
Thermal decomposition
[edit]When heated,n-BuLi, analogously to other alkyllithium reagents with "β-hydrogens", undergoesβ-hydride eliminationto produce1-buteneandlithium hydride(LiH):
- C4H9Li → LiH + CH3CH2CH=CH2
Safety
[edit]Alkyl-lithium compounds are stored under inert gas to prevent loss of activity and for reasons of safety.n-BuLi reacts violently with water:
- C4H9Li + H2O → C4H10+LiOH
This is an exergonic and highly exothermic reaction. If oxygen is present the butane produced may ignite.
BuLi also reacts with CO2to give lithium pentanoate:
- C4H9Li + CO2→ C4H9CO2Li
See also
[edit]- Propynyllithium,an organometallic compound.
References
[edit]- ^Bernier, David."Some useful pKa values".Org@Work.Archived fromthe originalon 9 May 2017.Retrieved26 May2017.
- ^Schwindeman, James A. (1 August 2014). "Preparation, Properties, and Safe Handling of Commercial Organolithiums: Alkyllithiums, Lithium sec-Organoamides, and Lithium Alkoxides".Organic Process Research & Development.18(10): 1192–1210.doi:10.1021/op500161b.
- ^abcdBrandsma, L.; Verkruijsse, H. D. (1987).Preparative Polar Organometallic Chemistry I.Berlin:Springer-Verlag.ISBN3-540-16916-4..
- ^"n-Butyllithium solution".sigmaaldrich.Retrieved17 August2023.
- ^Elschenbroich, C.” Organometallics” (2006) Wiley-VCH: Weinheim.ISBN3-527-29390-6.
- ^Juaristi, E.; Martínez-Richa, A.; García-Rivera, A.; Cruz-Sánchez, J. S. (1983). "Use of 4-Biphenylmethanol, 4-Biphenylacetic Acid and 4-Biphenylcarboxylic Acid/Triphenylmethane as Indicators in the Titration of Lithium Alkyls. Study of the Dianion of 4-Biphenylmethanol".The Journal of Organic Chemistry.48(15): 2603–2606.doi:10.1021/jo00163a038.
- ^Ulrich Wietelmann and Richard J. Bauer "Lithium and Lithium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a15_393.
- ^Delaney, M. S. (20 January 1991). "The rate of ethylene polymerization initiated by various chelating tertiary diamine: n‐butyllithium complexes".Journal of Applied Polymer Science.42(2): 533–541.doi:10.1002/app.1991.070420226.
- ^Sanders, R.; Mueller-Westerhoff, U. T. (1996). "The Lithiation of Ferrocene and Ruthenocene — A Retraction and an Improvement".Journal of Organometallic Chemistry.512(1–2): 219–224.doi:10.1016/0022-328X(95)05914-B.
- ^Schlosser, Manfred; Strunk, Sven (January 1984)."The" super-basic "butyllithium/potassium tert-butoxide mixture and other lickor-reagents".Tetrahedron Letters.25(7): 741–744.doi:10.1016/S0040-4039(01)80014-9.
- ^Clayden, Jonathan; Yasin, Samreen A. (11 February 2002)."Pathways for decomposition of THF by organolithiums: the role of HMPA".New Journal of Chemistry.26(2): 191–192.doi:10.1039/B109604D.ISSN1369-9261.
Further reading
[edit]- FMC Lithium manufacturer's product sheets
- Environmental Chemistry directory
- Weissenbacher, Anderson, Ishikawa,Organometallics,July 1998, p681.7002, Chemicals Economics Handbook SRI International
- HPV test plan, submitted by FMC Lithium to EPA
- Ovaska, T. V.e-EROSEncyclopedia of Reagents for Organic Synthesis"n-Butyllithium. "Wiley and sons. 2006.doi:10.1002/047084289X.rb395
- Greenwood, N. N.; Earnshaw, A.Chemistry of the Elements,2nd ed. 1997: Butterworth-Heinemann, Boston.