Amine oxide

Inchemistry,anamine oxide,also known as anamineN-oxideor simplyN-oxide,is achemical compoundthat has thechemical formulaR3N+−O−.It contains anitrogen-oxygencoordinate covalent bondwith three additional hydrogen and/orsubstituent-groups attached to nitrogen. Sometimes it is written asR3N→Oor, alternatively,[1]asR3N=O.
In the strict sense, the termamine oxideapplies only tooxidesof tertiaryamines.Sometimes it is also used for the analogous derivatives of primary and secondary amines.
Examples of amine oxides includepyridine-N-oxide,a water-soluble crystalline solid withmelting point62–67 °C, andN-methylmorpholineN-oxide,which is an oxidant.
Applications
[edit]Amine oxides are surfactants commonly used in consumer products such as shampoos, conditioners, detergents, and hard surface cleaners.[2]Alkyl dimethyl amine oxide (chain lengths C10–C16) is the most commercially used amine oxide.[3]They are considered a high production volume class of compounds in more than one member country of the Organisation for Economic Co-operation and Development (OECD); with annual production over 26,000, 16,000 and 6,800 tonnes (28,700, 17,600 and 7,500 short tons) in the US, Europe, and Japan, respectively.[2]In North America, more than 95% of amine oxides are used in home cleaning products.[4]They serve as stabilizers, thickeners, emollients, emulsifiers, and conditioners with active concentrations in the range of 0.1–10%.[2]The remainder (< 5%) is used in personal care, institutional, commercial products[5]and for unique patented uses such as photography.[2]
Properties
[edit]Amine oxides are used asprotecting groupfor amines and aschemicalintermediates. Long-chainalkylamine oxides are used asamphotericsurfactantsandfoamstabilizers.
Amine oxides are highlypolar moleculesand have apolarityclose to that ofquaternary ammonium salts.Small amine oxides are veryhydrophilicand have an excellentwatersolubilityand a very poor solubility in most organicsolvents.
Amine oxides are weakbaseswith apKbof around 4.5 that formR3N+−OH,cationichydroxylamines,uponprotonationat apHbelow their pKb.
Synthesis
[edit]Almost all amine oxides are prepared by theoxidationof either tertiary aliphatic amines or aromaticN-heterocycles.Hydrogen peroxideis the most common reagent both industrially and in academia, howeverperacidsare also important.[6]More specialised oxidising agents can see niche use, for instanceCaro's acidormCPBA.Spontaneous or catalysed reactions using molecular oxygen are rare. Certain other reactions will also produce amine oxides, such as theretro-Cope elimination,however they are rarely employed.
Reactions
[edit]Amine oxides exhibit many kinds of reactions.[7]
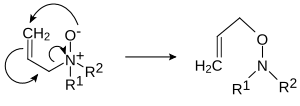
- Pyrolytic elimination. Amine oxides, when heated to 150–200 °C undergo aCope reactionto form a hydroxylamine and analkene.The reaction requires the alkyl groups to have hydrogens at the beta-carbon (i.e. works with ethyl and above, but not methyl)
- Reduction to amines. Amine oxides are readily converted to the parent amine by commonreductionreagentsincludinglithium aluminium hydride,sodium borohydride,catalytic reduction,zinc/acetic acid,and iron / acetic acid. PyridineN-oxides can be deoxygenated byphosphorus oxychloride
- Sacrificial catalysis. Oxidants can be regenerated by reduction ofN-oxides, as in the case of regeneration ofosmium tetroxidebyN-methylmorpholineN-oxide in theUpjohn dihydroxylation.
- O-Alkylation. PyridineN-oxides react withalkyl halidesto theO-alkylated product
- Bis-ter-pyridine derivatives adsorbed on silver surfaces are discussed to react with oxygen to bis-ter-pyridineN-oxide. This reaction can be followed by video-scanning tunneling microscopywith sub-molecular resolution.[8]
- In theMeisenheimer rearrangement(afterJakob Meisenheimer) certainN-oxidesR1R2R3N+−O−rearrangetohydroxylaminesR2R3N−O−R1[9][10]
- In thePolonovski reactiona tertiaryN-oxide is cleaved byacetic acid anhydrideto the correspondingacetamideandaldehyde:[11][12][13]
Metabolites
[edit]Amine oxides are commonmetabolitesof medication andpsychoactive drugs.Examples includenicotine,Zolmitriptan,andmorphine.
Amine oxides ofanti-cancer drugshave been developed asprodrugsthat are metabolized in theoxygen-deficient cancertissueto the active drug.
Human safety
[edit]Amine oxides (AO) are not known to be carcinogens, dermal sensitizers, or reproductive toxicants. They are readily metabolized and excreted if ingested. Chronic ingestion by rabbits found lower body weight, diarrhea, and lenticular opacities at a lowest observed adverse effect levels (LOAEL) in the range of 87–150 mg AO/kw bw/day. Tests of human skin exposure have found that after 8 hours less than 1% is absorbed into the body. Eye irritation due to amine oxides and other surfactants is moderate and temporary with no lasting effects.[2]
Environmental safety
[edit]Amine oxides with an average chain length of 12.6 have been measured to be water-soluble at ~410 g/L. They are considered to have lowbioaccumulationpotential in aquatic species based on logKowdata from chain lengths less than C14 (bioconcentration factor < 87%).[2]Levels of AO in untreated influent were found to be 2.3–27.8 ug/L, while in effluent they were found to be 0.4–2.91 ug/L. The highest effluent concentrations were found in oxidation ditch and trickling filter treatment plants. On average, over 96% removal has been found with secondary activated sludge treatment.[3]Acute toxicity in fish, as indicated by 96h LC50 tests, is in the range of 1,000–3,000 ug/L for carbon chain lengths less than C14. LC50 values for chain lengths greater than C14 range from 600 to 1400 ug/L. Chronic toxicity data for fish is 420 ug/L. When normalized to C12.9, the NOEC is 310 ug/L for growth and hatchability.[3]
See also
[edit]- Functional group
- Amine,NR3
- Hydroxylamine,NR2OH
- Phosphine oxide,PR3=O
- Sulfoxide,R2S=O
- Azoxy,RN=N+(O−)R RN=N+RO−
- Aminoxyl group,radicals with the general structure R2N–O•
- Category:Amine oxides,containing all articles on specific amine-oxide compounds
References
[edit]- ^Durrant, Marcus C. (2015)."A quantitative definition of hypervalency".Chemical Science.6(11): 6614–6623.doi:10.1039/C5SC02076J.PMC6054109.PMID30090275.
- ^abcdefOrganisation for Economic Co-operation and Development (OECD) (2006)."Amine Oxides".OECD Existing Chemicals Database.Archived fromthe originalon 22 February 2014.
- ^abcSanderson, H; C Tibazarwa; W Greggs; DJ Versteeg (2009). "High Production Volume Chemical Amine Oxides [C8–C20] ".Risk Analysis.29(6): 857–867.doi:10.1111/j.1539-6924.2009.01208.x.PMID19504658.S2CID45774397.
- ^Modler, RF; Inoguchi Y (2004)."CEH Marketing Research Report: Surfactants, Household Detergents, and their Raw Materials".Chemical Economics Handbook.Menlo Park, CA: SRI Consulting.
- ^Sanderson, H; Counts JL; Stanton K; Sedlak R (2006)."Exposure and Prioritization—Human Screening Data and Methods for High Production Volume Chemicals in Consumer Products: Amine Oxides a Case Study".Risk Analysis.26(6): 1637–1657.doi:10.1111/j.1539-6924.2006.00829.x.PMID17184403.
- ^Smith, Michael B.;March, Jerry(2007),Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(6th ed.), New York: Wiley-Interscience, p. 1779,ISBN978-0-471-72091-1
- ^Albini, Angelo (1993). "Synthetic utility of amineN-oxides ".Synthesis.1993(3): 263–77.doi:10.1055/s-1993-25843.
- ^Waldmann, T.; et al. (2012). "Oxidation of an Organic Adlayer: A Bird's Eye View".Journal of the American Chemical Society.134(21): 8817–22.doi:10.1021/ja302593v.PMID22571820.
- ^J. Meisenheimer, Ber. 52. 1667 (1919)
- ^Smith, Michael B.; March, Jerry (2001).March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(5th ed.). Wiley-Interscience.ISBN0-471-58589-0.[page needed]
- ^Grierson, D (1990). "The Polonovski Reaction".Org. React.39:85.doi:10.1002/0471264180.or039.02.ISBN0471264180.
- ^Polonovski, Max; Polonovski, Michel (1927). ""Sur les aminoxydes des alcaloïdes. III. Action des anhydrides et chlorures d'acides organiques. Préparation des bases nor."".Bull. Soc. Chim. Fr.41:1190–1208.
- ^Kürti, Laszlo; Czako, Barbara (2005).Strategic Applications of Named Reactions in Organic Synthesis(paperback ed.). Elsevier Science.ISBN0-12-429785-4.[page needed]