Nafion

| |
| Identifiers | |
|---|---|
| ChemSpider |
|
PubChemCID
|
|
CompTox Dashboard(EPA)
|
|
| Properties | |
| C7HF13O5S. C2F4 | |
| Molar mass | See Article |
| Hazards | |
| GHSlabelling: | |

| |
| Warning | |
| H319,H335 | |
| P261,P264,P271,P280,P304+P340,P305+P351+P338,P312,P337+P313,P403+P233,P405,P501 | |
| Related compounds | |
Related compounds
|
Aciplex Flemion Dowex fumapem F |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Nafionis a brand name for a sulfonatedtetrafluoroethylenebasedfluoropolymer-copolymersynthesized in 1962 by Dr. Donald J. Connolly at the DuPont Experimental Station in Wilmington Delaware (U.S. Patent 3,282,875). Additional work on the polymer family was performed in the late 1960s by Dr. Walther Grot ofDuPont.[1]Nafion is a brand of theChemourscompany. It is the first of a class of synthetic polymers with ionic properties that are calledionomers.Nafion's unique ionic properties are a result of incorporating perfluorovinyl ether groups terminated with sulfonate groups onto a tetrafluoroethylene (PTFE) backbone.[2][3][4]Nafion has received a considerable amount of attention as aproton conductorforproton exchange membrane (PEM) fuel cellsbecause of its excellent chemical and mechanical stability in the harsh conditions of this application.
The chemical basis of Nafion's ion-conductive properties remain a focus of extensive research.[2]Ion conductivity of Nafion increases with the level of hydration. Exposure of Nafion to a humidified environment or liquid water increases the amount of water molecules associated with each sulfonic acid group. The hydrophilic nature of the ionic groups attract water molecules, which begin to solvate the ionic groups and dissociate the protons from the -SO3H (sulfonic acid) group. The dissociated protons "hop" from one acid site to another throughmechanismsfacilitated by the water molecules andhydrogen bonding.[2]Upon hydration, Nafion phase-separates at nanometer length scales resulting in formation of an interconnected network of hydrophilic domains which allow movement of water andcations,but themembranesdo not conductelectronsand minimally conductanionsdue to permselectivity (charge-based exclusion). Nafion can be manufactured with or exchanged to alternate cation forms for different applications (e.g. lithiated for Li-ion batteries) and at different equivalent weights (EWs), alternatively considered as ion-exchange capacities (IECs), to achieve a range of cationic conductivities with trade-offs to other physicochemical properties such as water uptake and swelling.
Nomenclature and molecular weight[edit]
Nafion can be produced as both a powderresinand acopolymer.It has various chemical configurations and thus several chemical names in theIUPACsystem. Nafion-H, for example, includes the following systematic names:
- FromChemical Abstracts:ethanesulfonyl fluoride, 2-[1-[difluoro-[(trifluoroethenyl)oxy]methyl]-1,2,2,2-tetrafluoroethoxy]-1,1,2,2,-tetrafluoro-, with tetrafluoroethylene
- tetrafluoroethylene-perfluoro-3,6-dioxa-4-methyl-7-octenesulfonic acid copolymer
Themolecular weightof Nafion is variable due to differences in processing and solution morphology.[3][4]The structure of a Nafion unit illustrates the variability of the material; for example, the most basicmonomercontains chain variation between theethergroups (the z subscript). Conventional methods of determining molecular weight such aslight scatteringandgel permeation chromatographyare not applicable because Nafion is insoluble, although the molecular weight has been estimated at 105–106Da.[3][4]Instead, the equivalent weight (EW) and material thickness are used to describe most commercially available membranes. The EW is the number of grams of dry Nafion per mole of sulfonic acid groups when the material is in the acid form.[4]Nafion membranes are commonly categorized in terms of their EW and thickness.[2][5]For example, Nafion 117 indicates an extrusion-cast membrane with 1100 g/mol EW and 0.007 inches (7 thou) in thickness.[5]In contrast to equivalent weight, conventionalion-exchange resinsare usually described in terms of theirion exchangecapacity (IEC), which is the multiplicative inverse or reciprocal of the equivalent weight, i.e., IEC = 1000/EW.
Preparation[edit]
Nafion derivatives are first synthesized by the copolymerization oftetrafluoroethylene(TFE) (the monomer in Teflon) and a derivative of a perfluoro (alkyl vinyl ether) with sulfonyl acid fluoride. The latter reagent can be prepared by thepyrolysisof its respectiveoxideorcarboxylic acidto give the olefinated structure.[6]
The resulting product is an -SO2F-containingthermoplasticthat isextrudedinto films. Hot aqueous NaOH converts these sulfonyl fluoride (-SO2F) groups into sulfonate groups (-SO3−Na+). This form of Nafion, referred to as the neutral or salt form, is finally converted to the acid form containing the sulfonic acid (-SO3H) groups. Nafion can be dispersed into solution by heating in aqueous alcohol at 250 °C in anautoclavefor subsequent casting intothin filmsor use as polymeric binder in electrodes. By this process, Nafion can be used to generate composite films, coatelectrodes,or repair damaged membranes.[3]
Properties[edit]
The combination of the stable PTFE backbone with the acidic sulfonic groups gives Nafion its characteristics:[2][7]
- It is highly conductive to cations, making it suitable for many membrane applications.[2]
- It resists chemical attack. According to Chemours, onlyalkali metals(particularly sodium) can degrade Nafion under normal temperatures and pressures.
- The PTFE backbone interlaced with the ionic sulfonate groups gives Nafion a high chemical stability temperature (e.g. 190 °C) but a softening point in the range of 85-100 °C give it a moderateoperating temperature,e.g. up to 100 °C, with additional challenges in all applications due to the loss of water above 100 °C.
- It is asuperacidcatalyst. The combination of fluorinated backbone, sulfonic acid groups, and the stabilizing effect of the polymer matrix make Nafion a very strong acid, with pKa~ -6.[8]In this respect Nafion resembles thetrifluoromethanesulfonic acid,CF3SO3H, although Nafion is a weaker acid by at least three orders of magnitude.
- It is selectively and highly permeable to water.
- Its proton conductivity up to 0.2S/cm depending on temperature, hydration state, thermal history and processing conditions.[9][2]
- The solid phase and the aqueous phase of Nafion are both permeable to gases,[10][11]which is a drawback for energy conversion devices such as artificial leaves, fuel cells, and water electrolyzers.
Structure/morphology[edit]
The morphology of Nafion membranes is a matter of continuing study to allow for greater control of its properties. Other properties such as water management, hydration stability at high temperatures,electro-osmotic drag,as well as the mechanical, thermal, and oxidative stability, are affected by the Nafion structure. A number of models have been proposed for the morphology of Nafion to explain its unique transport properties.[2]

The first model for Nafion, called thecluster-channelorcluster-network model,consisted of an equal distribution of sulfonate ion clusters (also described as 'invertedmicelles'[4]) with a 40Å(4nm) diameter held within a continuous fluorocarbon lattice. Narrow channels about 10 Å (1 nm) in diameter interconnect the clusters, which explains the transport properties.[3][4][12]
The difficulty in determining the exact structure of Nafion stems from inconsistent solubility and crystalline structure among its various derivatives. Advanced morphological models have included acore-shell modelwhere the ion-rich core is surrounded by an ion poor shell, arod modelwhere the sulfonic groups arrange into crystal-like rods, and asandwich modelwhere the polymer forms two layers whose sulfonic groups attract across an aqueous layer where transport occurs.[4]Consistency between the models include a network of ionic clusters; the models differ in the cluster geometry and distribution. Although no model has yet been determined fully correct, some scientists have demonstrated that as the membrane hydrates, Nafion's morphology transforms from the cluster-channel model to a rod-like model.[4]
A cylindrical-water channel model[13]was also proposed based on simulations of small-angle X-ray scattering data and solid state nuclear magnetic resonance studies. In this model, the sulfonic acid functional groups self-organize into arrays of hydrophilic water channels, each ~ 2.5 nm in diameter, through which small ions can be easily transported. Interspersed between the hydrophilic channels are hydrophobic polymer backbones that provide the observed mechanical stability. Many recent studies, however, favored a phase-separated nanostructure consisting of locally-flat, or ribbon-like, hydrophilic domains based on evidence from direct-imaging studies[14]and more comprehensive analysis of the structure and transport properties.[2][15]
Applications[edit]
Nafion's properties make it suitable for a broad range of applications. Nafion has found use infuel cells,electrochemical devices, chlor-alkali production, metal-ion recovery, waterelectrolysis,plating,surface treatment of metals, batteries,sensors,Donnan dialysis cells,drug release, gas drying or humidification, andsuperacidcatalysis for the production of fine chemicals.[3][4][7][16]Nafion is also often cited for theoretical potential (i.e., thus far untested) in a number of fields. With consideration of Nafion's wide functionality, only the most significant will be discussed below.
Chlor-alkali production cell membrane[edit]
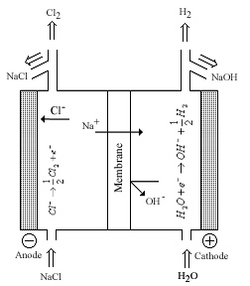
Chlorine and sodium/potassium hydroxide are among the most produced commodity chemicals in the world. Modern production methods produce Cl2and NaOH/KOH from the electrolysis ofbrineusing a Nafion membrane between half-cells. Before the use of Nafion, industries usedmercurycontaining sodium amalgam to separate sodium metal from cells orasbestosdiaphragms to allow for transfer of sodium ions between half cells; both technologies were developed in the latter half of the 19th century. The disadvantages of these systems is worker safety and environmental concerns associated with mercury and asbestos, economical factors also played a part, and in the diaphragm process chloride contamination of the hydroxide product. Nafion was the direct result of the chlor-alkali industry addressing these concerns; Nafion could tolerate the high temperatures, high electrical currents, and corrosive environment of the electrolytic cells.[3][4][7]
The figure to the right shows a chlor-alkali cell where Nafion functions as a membrane between half cells. The membrane allows sodium ions to transfer from one cell to the other with minimal electrical resistance. The membrane was also reinforced with additional membranes to prevent gas product mi xing and minimize back transfer of Cl−and−OH ions.[3]
Proton exchange membrane (PEM) for fuel cells[edit]
Although fuel cells have been used since the 1960s as power supplies for satellites, recently they have received renewed attention for their potential to efficiently produce clean energy from hydrogen. Nafion was found effective as a membrane forproton exchange membrane(PEM)fuel cellsby permitting hydrogen ion transport while preventing electron conduction. Solid Polymer Electrolytes, which are made by connecting or depositing electrodes (usually noble metal) to both sides of the membrane, conduct the electrons through an energy requiring process and rejoin the hydrogen ions to react with oxygen and produce water.[3]Fuel cells are expected to find strong use in the transportation industry.
Superacid catalyst for fine chemical production[edit]
Nafion, as asuperacid,has potential as a catalyst fororganic synthesis.Studies have demonstrated catalytic properties inalkylation,isomerization,oligomerization,acylation,ketalization,esterification,hydrolysisofsugarsandethers,andoxidation.New applications are constantly being discovered.[16]These processes, however, have not yet found strong commercial use. Several examples are shown below:
Alkylation with alkyl halides[edit]
Nafion-H gives efficient conversion whereas the alternative method, which employsFriedel-Crafts synthesis,can promote polyalkylation:[17]
Acylation[edit]
The amount of Nafion-H needed to catalyze the acylation of benzene with aroyl chloride is 10–30% less than the Friedel-Crafts catalyst:[17]
Catalysis of protection groups[edit]
Nafion-H increasesreaction ratesofprotectionvia dihydropyran or o-trialkylsilation of alcohols, phenol, and carboxylic acids.[16]
Isomerization[edit]
Nafion can catalyze a1,2-hydride shift.[16]
It is possible to immobilizeenzymeswithin the Nafion by enlarging pores withlipophilicsalts. Nafion maintains a structure and pH to provide a stable environment for the enzymes. Applications include catalytic oxidation ofadenine dinucleotides.[16]
Sensors[edit]
Nafion has found use in the production ofsensors,with application in ion-selective, metallized, optical, andbiosensors.What makes Nafion especially interesting is its demonstration inbiocompatibility.Nafion has been shown to be stable incell culturesas well as the human body, and there is considerable research towards the production of higher sensitivityglucosesensors.[3]
Antimicrobial surfaces[edit]
Nafion surfaces show an exclusion zone against bacteria colonization.[18]Moreover, layer-by-layer coatings comprising Nafion show excellent antimicrobial properties.[19]
Dehumidification in spacecraft[edit]
TheSpaceX Dragon 2human-rated spacecraft uses Nafion membranes to dehumidify the cabin air. One side of the membrane is exposed to the cabin atmosphere, the other to the vacuum of space. This results in dehumidification since Nafion is permeable to water molecules but not air. This saves power and complexity since cooling is not required (as needed with a condensing dehumidifier), and the removed water is rejected to space with no additional mechanism needed.[20]
Modified Nafion for PEM fuel cells[edit]
Normal Nafion will dehydrate (thus lose proton conductivity) when the temperature is above ~80 °C. This limitation troubles the design of fuel cells because higher temperatures are desirable for better efficiency and CO tolerance of the platinum catalyst. Silica and zirconium phosphate can be incorporated into Nafion water channels throughin situchemical reactions to increase the working temperature to above 100 °C.
References[edit]
- ^Church, Steven (January 6, 2006). "Del. firm installs fuel cell".The News Journal.p. B7.
- ^abcdefghiKusoglu, Ahmet; Weber, Adam Z. (2017-02-08)."New Insights into Perfluorinated Sulfonic-Acid Ionomers".Chemical Reviews.117(3): 987–1104.doi:10.1021/acs.chemrev.6b00159.ISSN0009-2665.PMID28112903.
- ^abcdefghijHeitner-Wirguin, C. (1996). "Recent advances in perfluorinated ionomer membranes: structure, properties and applications".Journal of Membrane Science.120:1–33.doi:10.1016/0376-7388(96)00155-X.
- ^abcdefghijMauritz, Kenneth A.; Moore, Robert B. (2004). "State of Understanding of Nafion".Chemical Reviews.104(10): 4535–4586.doi:10.1021/cr0207123.PMID15669162.
- ^ab"nafion membrane, chemours nafion, proton exchange membrane".nafion.Retrieved2021-04-22.
- ^Connolly, D.J.; Longwood; Gresham, W. F. (1966). "Fluorocarbon Vinyl Ether Polymers".Google Patents.U.S. patent 3,282,875.
- ^abcPerma Pure LLC (2004)."Nafion: Physical and Chemical Properties".Technical Notes and Articles.Archived fromthe originalon September 28, 2013.
- ^Schuster, M., Ise, M., Fuchs, A., Kreuer, K.D., Maier, J. (2005)."Proton and Water Transport in Nano-separated Polymer Membranes"(PDF).Le Journal de Physique IV.10.Germany: Max-Planck-Institut für Festkörperforschung: Pr7-279-Pr7-281.doi:10.1051/jp4:2000756.ISSN1155-4339.Archived from the original on 2007-06-11.
{{cite journal}}:CS1 maint: bot: original URL status unknown (link) CS1 maint: multiple names: authors list (link) - ^Sone, Yoshitsugu; Ekdunge, Per; Simonsson, Daniel (1996-04-01)."Proton Conductivity of Nafion 117 as Measured by a Four-Electrode AC Impedance Method".Journal of the Electrochemical Society.143(4): 1254.Bibcode:1996JElS..143.1254S.doi:10.1149/1.1836625.ISSN1945-7111.
- ^Schalenbach, Maximilian; Hoefner, Tobias; Paciok, Paul; Carmo, Marcelo; Lueke, Wiebke; Stolten, Detlef (2015-10-28). "Gas Permeation through Nafion. Part 1: Measurements".The Journal of Physical Chemistry C.119(45): 25145–25155.doi:10.1021/acs.jpcc.5b04155.
- ^Schalenbach, Maximilian; Hoeh, Michael A.; Gostick, Jeff T.; Lueke, Wiebke; Stolten, Detlef (2015-10-14). "Gas Permeation through Nafion. Part 2: Resistor Network Model".The Journal of Physical Chemistry C.119(45): 25156–25169.doi:10.1021/acs.jpcc.5b04157.
- ^Gierke, T. D.; Munn, G. E.; Wilson, F. C. (1981). "The morphology in nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies".Journal of Polymer Science: Polymer Physics Edition.19(11): 1687–1704.Bibcode:1981JPoSB..19.1687G.doi:10.1002/pol.1981.180191103.
- ^Schmidt-Rohr, K.; Chen, Q. (2007). "Parallel cylindrical water nanochannels in Nafion fuel-cell membranes".Nature Materials.7(1): 75–83.doi:10.1038/nmat2074.PMID18066069.
- ^Allen, Frances I.; Comolli, Luis R.; Kusoglu, Ahmet; Modestino, Miguel A.; Minor, Andrew M.; Weber, Adam Z. (2015-01-20)."Morphology of Hydrated As-Cast Nafion Revealed through Cryo Electron Tomography".ACS Macro Letters.4(1): 1–5.doi:10.1021/mz500606h.ISSN2161-1653.PMID35596390.
- ^Kreuer, Klaus-Dieter; Portale, Giuseppe (2013-11-20)."A Critical Revision of the Nano-Morphology of Proton Conducting Ionomers and Polyelectrolytes for Fuel Cell Applications".Advanced Functional Materials.23(43): 5390–5397.doi:10.1002/adfm.201300376.S2CID94579140.
- ^abcdeGelbard, Georges (2005). "Organic Synthesis by Catalysis with Ion-Exchange Resins".Industrial & Engineering Chemistry Research.44(23): 8468–8498.doi:10.1021/ie0580405.
- ^abEl-Kattan, Y.; McAtee, J.; Nafion-H. (2001) "Nafion-H". InEncyclopedia of Reagents for Organic Synthesis.John Wiley & Sons,ISBN978-0-470-01754-8.
- ^Cheng, Yifan; Moraru, Carmen I. (2018)."Long-range interactions keep bacterial cells from liquid-solid interfaces: Evidence of a bacteria exclusion zone near Nafion surfaces and possible implications for bacterial attachment".ColloidsSurf. B: Biointerfaces.162:16–24.doi:10.1016/j.colsurfb.2017.11.016.PMID29132042.
- ^Gibbons, Ella N.; Winder, Charis; Barron, Elliot; et al. (2019)."Layer by Layer Antimicrobial Coatings Based on Nafion, Lysozyme, and Chitosan".Nanomaterials.9(1563): 1563.doi:10.3390/nano9111563.PMC6915488.PMID31689966.
- ^Jason Silverman; Andrew Irby; Theodore Agerton (2020).Development of the Crew Dragon ECLSS(PDF).International Conference on Environmental Systems.
External links[edit]
- What Nafion Membrane is Right for an Electrolyzer / Hydrogen Generation?
- Homepage of Walther G. Grot
- Walther G. Grot: "Fluorinated Ionomers"
- Isotopic effects on Nafion conductivity
- Membrane thickness on conductivity_of_Nafion
- Nafion hydration
- Nafion Totally Explainedat theWayback Machine(archived 22 September 2007)




