Olfactory ensheathing cell

Olfactory ensheathing cells(OECs), also known asolfactory ensheathing gliaorolfactory ensheathing glial cells,are a type ofmacroglia(radial glia) found in thenervous system.They are also known asolfactorySchwann cells,because they ensheath the non-myelinatedaxonsofolfactory neuronsin a similar way to which Schwann cells ensheath non-myelinatedperipheral neurons.They also share the property of assisting axonal regeneration.
OECs are capable ofphagocytosingaxonal debrisin vivo,andin vitrothey phagocytosebacteria.Olfactory glia that express theantimicrobialenzymelysozyme(LYZ) are thought to play an important role inimmunoprotectionin themucosa,whereneuronsare directly exposed to the external environment.
OECs have been tested successfully in experimental axonal regeneration in adult rats withtraumatic spinal cord damage,andclinical trialsare currently being conducted to obtain more information on spinal cord injuries and otherneurodegenerativediseases.
Origin
[edit]
In theperipheral nervous systemOECs are dispersed within theolfactory epitheliumand theolfactory nerve.In thecentral nervous system,OECs are found within the outer two layers of theolfactory bulb.During development, primitive olfactory neurons extend their axons from theolfactory placode,through themesenchyme,towards the telencephalic vesicle.[1]After reaching thetelencephalic vesicle,a small layer of cells and axons cover the vesicle. Olfactory axons invade thebasal laminaof theglia limitansand theolfactory bulbto create the olfactory nerve andglomerular layers.A fraction of the epithelial migrating precursors give rise to olfactory ensheathing glia that inhabit the olfactory nerve and glomerular layers.[1]OECs andastrocytesinteract with each other to form a newglia limitans.[1]OECs are distinct from other glia in their developmental origin for they are present in the peripheral nervous system as well as the central nervous system. They also form on bundles of olfactory sensory neuron axons in a manner distinct frommyelination.
Functions
[edit]OECs are radial glia that perform a variety of functions. Within the olfactory system they phagocytose axonal debris and dead cells. Whenculturedin apetri dish(in vitro), they phagocytose bacteria. Multiple studies have shown that OECs may assist in treatingspinal cord injury(SCI) due to their regenerate properties in the peripheral nervous system and their presence in the central nervous system.[2]OECs are also known to support and guide olfactory axons, grow through glial scars, and secrete manyneurotrophic factors.[3]
OECs expressglial markerssuch asglial fibrillary acidic protein,s100,andp75,and radial glial markers such asnestinandvimentin,which may further assist researchers with understanding the labeling characteristics of these specialized glia.
Olfactory system regeneration
[edit]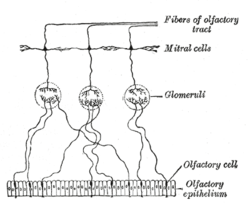
The mammalianolfactory systemis unusual in that it has the ability to continuously regenerate its neurons during adulthood.[4]This ability is associated with olfactory ensheathing glia. Newolfactory receptor neuronsmust project their axons through the central nervous system to anolfactory bulbin order to be functional. The growth and regeneration of olfactory axons can be attributable to OECs, as they form the fascicles through which axons grow from the peripheral nervous system into the central nervous system.[5]Olfactory receptor neurons have an average lifespan of 6–8 weeks and therefore must be replaced by cells differentiated from the stem cells that are within a layer at the nearby epithelium's base.Axonal growthis guided by the glial composition and cytoarchitecture of the olfactory bulb in addition to the presence of OECs.[4]
OECs are thought to be in part responsible for the neurogenesis of primary olfactory neurons through the processes offasciculation,cell sorting,and axonal targeting.[6]
Role in spinal cord injuries
[edit]Traumatic spinal cord damagecauses a permanent loss of motor and sensory functions in the central nervous system, termedparaplegiaortetraplegiabased on the site of the injury. Other detrimental effects may take place in therespiratory systemandrenal systemas a result of the injury. Unlike the peripheral nervous system, the central nervous system is unable to regenerate damaged axons, so its synaptic connections are lost forever. Current treatment is limited and the primary potential methods are either controversial or noneffective. Studies dating back to the 1990s have begun researching the olfactory system of mammals, rats in particular, to gain a greater understanding of axonal regeneration andneurogenesis,and the possible implementation of these cells at the site of the spinal cord injury.
Transplantation of OECs into the spinal cord has become a possible therapy for spinal cord damage and other neural diseases in animal models. Several recent studies have reported that preventing OEC inhibition will present a uniform population of cells in the spinal cord, creating an environment in which damaged axons can be repaired. In October 2014, the Polish firefighterDarek Fidykabecame the first paraplegic patient to regain mobility after OEC transplantation.[7][8]
OECs are similar to Schwann cells in that they provide an upregulation of low-affinityNGF receptor p75following injury; however, unlike Schwann cells they produce lower levels ofneurotrophins.Several studies have shown evidence of OECs being able to support regeneration of lesioned axons, but these results are often unable to be reproduced.[4]Regardless, OECs have been investigated thoroughly in relation to spinal cord injuries,amyotrophic lateral sclerosis,and other neurodegenerative diseases. Researchers suggest that these cells possess a unique ability to remyelinate injured neurons.[9]
Peptide-modified gellan gum and OECs
[edit]Stem cell transplantationhas been identified as another possible therapy for axonal regeneration in the central nervous system by delivering these cells directly to the site of the spinal cord injury. Both OECs and neural stem/progenitor cells (NSPCs) have been successfully transplanted in the central nervous system of adult rats and have had either positive or neutral results as a method of neurogenesis and axonal regeneration; however, neither method has been shown to have long term beneficial effects, as cell survival is usually less than 1% after transplantation.[3]The inability of these cells to sustain after transplantation is a result ofinflammation,the inability of a sufficient matrix to thrive and create a uniform population of cells, or the migratory response of the cells needed to fully repair the site of the injury. Another current issue with the survival of the cells is utilizing the properbiomaterialsto deliver them to the site of the injury.
One study has investigated the use of peptide modifiedgellan gumas the biomaterial with OECs andneural stem/progenitor cellsto provide an environment that will allow these cells to survive after transplantation.[3]Gellan gum hydrogel can be injected in a minimallyinvasivemanner and is approved by the FDA as a food additive because of its chemical structure. The gellan gum was modified with severalfibronectin-derived peptide sequences so the transplantation cells have closely related properties to that of native tissue in theextracellular matrix.[3]By mimicking native tissue, the delivery cells are less likely to be rejected by the body and biological functions such as cell adhesion and growth will be enhanced through cell-cell and cell-matrix interactions. In order to determine the possibility of OECs and NPSCs improving cell viability, both cells wereco-culturedin direct contact with each other, along with the peptide-modified gellan gum.[3]
The experiment demonstrated that NSPC adhesion, proliferation, and viability are greatly increased when the peptide-modified gellan gum is used as the transplantation device when compared to a gellan gum control.[3]Additionally, the co-culture of OECs and NSPCs shows greater cell survival compared to the cell survival of NSPCs cultured alone. The results provide evidence that this method of cell transplantation is a potential strategy for repairing spinal cord damage in the future.
Side effects of cell transplantation
[edit]A study has shown that cell transplantation may cause an increase in body temperature of a subject with an older injury to the spinal cord. In this experiment, the patients' body temperatures were elevated to those of a moderatefeverafter transplantation, and lasted approximately 3–4 days. However, the study provides evidence that even past spinal cord injuries can benefit from the neurological functional recovery that stem cell transplantation may provide in the future.[10]
Transplantation of stem cells is also known to causetoxicityandgraft-versus-host disease(GVHD).Apoptotic cellshave been administered simultaneously with hematopoietic stem cells in experimental transplantation models, in anticipation of an improved outcome.[11]As a result, the combination preventsalloimmunization,up-regulatesRegulatory T cells(suppressor T cells) and reduces the severity of GVHD.[11]
Infection susceptibility
[edit]OECs have properties similar to those ofastrocytes,[12]both of which have been identified as being susceptible to viral infection.[9][12]
Labeling OECs
[edit]Iron oxide particles for MRI
[edit]As stem cell transplantation is becoming a more prevalent means of treating traumatic spinal cord damage, many processes between the start and end result need to be addressed and made more efficient. By labeling OECs, these cells can be tracked by amagnetic resonance imaging(MRI) device when being dispersed in the central nervous system[13]A recent study made use of a novel type ofmicron-sized particles of iron oxide(MPIO) to label and track these transport-mediated cells via MRI.[13]The experiment resulted in an OEC labeling efficiency of more than 90% with an MPIOincubationtime as short as 6 hours, without affectingcell proliferation,migration and viability.[13]MPIOs have also been successfully transplanted into thevitreousbody of adult rat eyes, providing the first detailed protocol for efficient and safe MPIO labeling of OECs for their non-invasive MRI tracking in real time for use in studies of central nervous system repair and axonal regeneration.[13]
Subpopulations
[edit]Two distinct subpopulations of OECs have been identified[14]with high or low cell surface expression oflow-affinity nerve growth factor receptor(p75).
See also
[edit]- Neuroscience
- Neuroglia
- Olfaction
- Olfactory system
- Microglia
- List of distinct cell types in the adult human body
References
[edit]- ^abcRamón-Cueto A, Avila J (June 1998). "Olfactory ensheathing glia: properties and function".Brain Research Bulletin.46(3): 175–87.doi:10.1016/s0361-9230(97)00463-2.PMID9667810.S2CID8527441.
- ^Nocentini S, Reginensi D, Garcia S, Carulla P, Moreno-Flores MT, Wandosell F, et al. (May 2012). "Myelin-associated proteins block the migration of olfactory ensheathing cells: an in vitro study using single-cell tracking and traction force microscopy".Cellular and Molecular Life Sciences.69(10): 1689–703.doi:10.1007/s00018-011-0893-1.hdl:2445/36438.PMID22205212.S2CID6548351.
- ^abcdefSilva NA, Cooke MJ, Tam RY, Sousa N, Salgado AJ, Reis RL, Shoichet MS (September 2012). "The effects of peptide modified gellan gum and olfactory ensheathing glia cells on neural stem/progenitor cell fate".Biomaterials.33(27): 6345–54.doi:10.1016/j.biomaterials.2012.05.050.hdl:1822/20032.PMID22698724.
- ^abcRuitenberg MJ, Vukovic J, Sarich J, Busfield SJ, Plant GW (March–April 2006). "Olfactory ensheathing cells: characteristics, genetic engineering, and therapeutic potential".Journal of Neurotrauma.23(3–4): 468–78.doi:10.1089/neu.2006.23.468.PMID16629630.
- ^Chehrehasa F, Ekberg JA, Lineburg K, Amaya D, Mackay-Sim A, St John JA (February 2012). "Two phases of replacement replenish the olfactory ensheathing cell population after injury in postnatal mice".Glia.60(2): 322–32.doi:10.1002/glia.22267.hdl:10072/45582.PMID22065423.S2CID7490951.
- ^Windus LC, Lineburg KE, Scott SE, Claxton C, Mackay-Sim A, Key B, St John JA (May 2010)."Lamellipodia mediate the heterogeneity of central olfactory ensheathing cell interactions".Cellular and Molecular Life Sciences.67(10): 1735–50.doi:10.1007/s00018-010-0280-3.PMC11115728.PMID20143249.S2CID25048015.
- ^Quinn B (21 October 2014)."Paralysed man Darek Fidyka walks again after pioneering surgery".The Guardian.Retrieved14 February2015.
[Fidyka], who is believed to be the first person in the world to recover from complete severing of the spinal nerves, can now walk with a frame and has been able to resume an independent life, even to the extent of driving a car, while sensation has returned to his lower limbs.
- ^"Paralysed man walks again after cell treatment".BBC.21 October 2014.Retrieved14 February2015.
- ^abHarberts E, Yao K, Wohler JE, Maric D, Ohayon J, Henkin R, Jacobson S (August 2011)."Human herpesvirus-6 entry into the central nervous system through the olfactory pathway".Proceedings of the National Academy of Sciences of the United States of America.108(33): 13734–9.Bibcode:2011PNAS..10813734H.doi:10.1073/pnas.1105143108.PMC3158203.PMID21825120.
- ^Liu C, Zheng Z, Gao R, Zhang K, Zhang L, Zhang L, Zhang L, Wei S, Kuang N, Song Y (2008). "Influence of olfactory ensheathing cell transplantation on body temperature of patients with old spinal cord injury".Neural Regeneration Research.3(7): 805–808.
- ^abPessach I, Shimoni A, Nagler A (November 2012). "Apoptotic cells in allogeneic hematopoietic stem cell transplantations:" turning trash into gold "".Leukemia & Lymphoma.53(11): 2130–5.doi:10.3109/10428194.2012.690099.PMID22553946.S2CID29417980.
- ^abCassiani-Ingoni R, Greenstone HL, Donati D, Fogdell-Hahn A, Martinelli E, Refai D, et al. (November 2005)."CD46 on glial cells can function as a receptor for viral glycoprotein-mediated cell-cell fusion".Glia.52(3): 252–8.doi:10.1002/glia.20219.PMID15920733.S2CID25598238.
- ^abcdSandvig I, Hoang L, Sardella TC, Barnett SC, Brekken C, Tvedt K, et al. (2012). "Labelling of olfactory ensheathing cells with micron-sized particles of iron oxide and detection by MRI".Contrast Media & Molecular Imaging.7(4): 403–10.doi:10.1002/cmmi.1465.hdl:11250/2623054.PMID22649046.
- ^Honoré A, Le Corre S, Derambure C, Normand R, Duclos C, Boyer O, et al. (March 2012). "Isolation, characterization, and genetic profiling of subpopulations of olfactory ensheathing cells from the olfactory bulb".Glia.60(3): 404–13.doi:10.1002/glia.22274.PMID22161947.S2CID31230806.
