Organoid
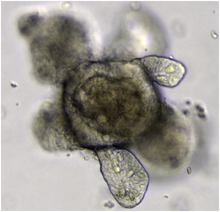
Anorganoidis a miniaturised and simplified version of anorganproducedin vitroin three dimensions that mimics the key functional, structural, and biological complexity of that organ.[1]It is derived from one or a fewcellsfrom atissue,embryonic stem cells,orinduced pluripotent stem cells,which canself-organizein three-dimensional culture owing to theirself-renewalanddifferentiationcapacities. The technique for growing organoids has rapidly improved since the early 2010s, andThe Scientistnamed it one of the biggest scientific advancements of 2013.[2]Scientists and engineers use organoids to study development and disease in thelaboratory,for drug discovery and development in industry,[3]personalized diagnostics and medicine, gene and cell therapies, tissue engineering, and regenerative medicine.
History
[edit]Attempts to createorgansin vitrostarted with one of the first dissociation-reaggregation experiments[4]whereHenry Van Peters Wilsondemonstrated that mechanically dissociated sponge cells can reaggregate and self-organize to generate a whole organism.[5]In the subsequent decades, multiple labs were able to generate different types of organs[4]in vitrothrough the dissociation and reaggregation of organ tissues obtained from amphibians[6]and embryonic chicks.[7]The formation of first tissue-like coloniesin vitrowas observed for the first time by co-culturingkeratinocytesand 3T3 fibroblasts.[8]The phenomena of mechanically dissociated cells aggregating and reorganizing to reform the tissue they were obtained from subsequently led to the development of thedifferential adhesion hypothesisbyMalcolm Steinberg.[4]With the advent of the field ofstem cellbiology, the potential of stem cells to form organsin vitrowas realized early on with the observation that when stem cells formteratomasorembryoid bodies,thedifferentiated cellscan organize into different structures resembling those found in multipletissuetypes.[4]The advent of the field of organoids, started with a shift from culturing and differentiating stem cells in two dimensional (2D) media, to three dimensional (3D) media to allow for the development of the complex 3-dimensional structures of organs.[4]Utilization of 3D media culture media methods for the structural organization was made possible with the development ofextracellular matrices(ECM).[9]In the late 1980s,Bisselland colleagues showed that a laminin rich gel can be used as a basement membrane for differentiation and morphogenesis in cell cultures of mammary epithelial cells.[10][11]Since 1987, researchers have devised different methods for 3D culturing, and were able to utilize different types of stem cells to generate organoids resembling a multitude of organs.[4]In the 1990s, in addition to their role in physical support, the role of ECM components in gene expression by their interaction with integrin-based focal adhesion pathways was reported.[12]In 2006,Yaakov Nahmiasand David Odde showed the self-assembly of vascularliverorganoid maintained for over 50 daysin vitro.[13]In 2008,Yoshiki Sasaiand his team atRIKENinstitute demonstrated thatstem cellscan be coaxed into balls ofneuralcells that self-organize into distinctive layers.[14]In 2009 the Laboratory ofHans CleversatHubrecht InstituteandUniversity Medical Center Utrecht,Netherlands, showed that singleLGR5-expressing intestinal stem cells self-organize to crypt-villus structuresin vitrowithout necessity of amesenchymalniche, making them the first organoids.[15]In 2010, Mathieu Unbekandt &Jamie A. Daviesdemonstrated the production of renal organoids from murine fetus-derived renogenic stem cells.[16]In 2014, Qun Wang and co-workers engineered collagen-I and laminin based gels and synthetic foam biomaterials for the culture and delivery of intestinal organoids[17]and encapsulated DNA-functionalized gold nanoparticles into intestinal organoids to form an intestinal Trojan horse for drug delivery and gene therapy.[18]Subsequent reports showed significant physiological function of these organoidsin vitro[19]andin vivo.[20][21]
Other significant early advancements included in 2013,Madeline Lancasterat theInstitute of Molecular Biotechnologyof theAustrian Academy of Sciencesestablished a protocol starting from pluripotent stem cells to generatecerebral organoidsthat mimic the developing human brain's cellular organization.[22]Meritxell Huchand Craig Dorrell atHubrecht InstituteandUniversity Medical Center Utrechtdemonstrated that single Lgr5+ cells from damaged mouse liver can be clonally expanded as liver organoids in Rspo1-based culture medium over several months.[23]In 2014, Artem Shkumatov et al. at the University of Illinois at Urbana-Champaign demonstrated that cardiovascular organoids can be formed fromES cellsthrough modulation of the substrate stiffness, to which they adhere. Physiological stiffness promoted three-dimensionality of EBs and cardiomyogenic differentiation.[24]
Properties
[edit]Lancaster and Knoblich[4]define an organoid as a collection of organ-specific cell types that develops from stem cells or organ progenitors, self-organizes through cell sorting and spatially restricted lineage commitment in a manner similar toin vivo,and exhibits the following properties:
- it has multiple organ-specific cell types;
- it is capable of recapitulating some specific function of the organ (e.g.contraction,neuralactivity,endocrinesecretion, filtration,excretion);
- its cells are grouped together and spatially organized, similar to an organ.
Process
[edit]Organoid formation generally requires culturing thestem cellsorprogenitor cellsin a 3D medium.[4]Stem cells have the ability to self-renew and differentiate into various cell subtypes, and they enable understanding the processes of development and disease progression.[25]Therefore organoids derived from stem cells enable studying biology and physiology at the organ level.[26]The 3D medium can be made using an extracellular matrixhydrogelsuch asMatrigelor Cultrex BME, which is alaminin-rich extracellular matrix that is secreted by the Engelbreth-Holm-Swarm tumor line.[27]Organoid bodies can then be made through embedding stem cells in the 3D medium.[4]Whenpluripotentstem cells are used for the creation of the organoid, the cells are usually, but not all the time, allowed to formembryoid bodies.[4]Those embryoid bodies are then pharmacologically treated with patterning factors to drive the formation of the desired organoid identity.[4]Organoids have also been created using adult stem cells extracted from the target organ, and cultured in 3D media.[28]
Biochemical cues have been incorporated in 3D organoid cultures and with exposure of morphogenes, morphogen inhibitors, or growth factors, organoid models can be developed using embryonic stem cells (ESCs) or adult stem cells (ASCs). Vascularization techniques can be utilized to embody microenvironments that are close to their counterparts, physiologically. Vasculature systems that can facilitate oxygen or nutrients to the inner mass of organoids can be achieved through microfluidic systems, vascular endothelial growth factor delivery systems, and endothelial cell-coated modules.[9]With patient-derivedinduced pluripotent stem cells(iPSCs)[29]andCRISPR/Cas-based genome editing[30]technologies, genome-edited or mutated pluripotent stem cells (PSCs) with altered signaling cues can be generated to control intrinsic cues within organoids.
Types
[edit]A multitude of organ structures have been recapitulated using organoids.[4]This section aims to outline the state of the field as of now through providing an abridged list of the organoids that have been successfully created, along with a brief outline based on the most recent literature for each organoid, and examples of how it has been utilized in research.
Cerebral organoid
[edit]Acerebral organoiddescribes artificially grown,in vitro,miniature organs resembling thebrain.Cerebral organoids are created by culturing humanpluripotent stem cellsin a three-dimensional structure using rotationalbioreactorand develop over the course of months.[22]The procedure has potential applications in the study of brain development, physiology and function. Cerebral organoids may experience "simple sensations" in response to external stimulation and neuroscientists are among those expressing concern that such organs could developsentience.They propose that further evolution of the technique needs to be subject to a rigorous oversight procedure.[31][32][33]In 2023, researchers have built a hybrid biocomputer that combines laboratory-grown human brain organoids with conventional circuits, and can complete tasks such as voice recognition.[34]Cerebral Organoids are currently being used to research and developOrganoid Intelligence (OI)technologies.[35]
Gastrointestinal organoid
[edit]Gastrointestinal organoids refer to organoids that recapitulate structures of thegastrointestinal tract.The gastrointestinal tract arises from theendoderm,which during development forms a tube that can be divided in three distinct regions, which give rise to, along with other organs, the following sections of the gastrointestinal tract:[4]
Organoids have been created for the following structures of the gastrointestinal tract:
Intestinal organoid
[edit]Intestinal organoids[15]have thus far been among the gut organoids generated directly from intestinal tissues or pluripotent stem cells.[4]One way human pluripotent stem cells can be driven to form intestinal organoids is through first the application ofactivinA to drive the cells into a mesoendodermal identity, followed by the pharmacological upregulation ofWnt3aandFgf4signaling pathways as they have been demonstrated to promote posterior gut fate.[4]Intestinal organoids have also been generated from intestinal stem cells, extracted from adult tissue and cultured in 3D media.[28]These adult stem cell-derived organoids are often referred to as enteroids or colonoids, depending on their segment of origin, and have been established from both the human and murine intestine.[15][36][37]Intestinal organoids consist of a single layer of polarized intestinalepithelialcells surrounding a central lumen. As such, recapitulate the crypt-villus structure of the intestine, by recapitulating its function, physiology and organization, and maintaining all the cell types found normally in the structure including intestinal stem cells.[4]Thus, intestinal organoids are a valuable model to study intestinal nutrient transport,[38][39]drug absorption and delivery,[40][41]nanomaterialsand nanomedicine,[42][43]incretin hormone secretion,[44][45]and infection by various enteropathogens.[46][47]For example, Qun Wang's team rationally designed artificial virus nanoparticles as oral drug delivery vehicles (ODDVs) with gut organoid-derived mucosal models[48]and demonstrated a new concept of using newly established colon organoids as tools for high-throughput drug screening, toxicity testing, and oral drug development.[49]Intestinal organoids also recapitulate thecrypt-Villusstructure to such a high degree of fidelity that they have been successfully transplanted to mouse intestines, and are hence highly regarded as a valuable model for research.[4]One of the fields of research that intestinal organoids have been utilized is that of stem cell niche. Intestinal organoids were used to study the nature of theintestinal stem cell niche,and research done with them demonstrated the positive roleIL-22has in maintaining in intestinal stem cells,[50]along with demonstrating the roles of other cell types like neurons and fibroblasts in maintenance of intestinal stem cells.[28]In the field of infection biology, different intestinal organoid-based model systems have been explored. On one hand, organoids can be infected in bulk by simply mi xing them with theenteropathogenof interest.[51]However, to model infection via a more natural route starting from the intestinal lumen, microinjection of thepathogenis required.[52][53]In addition, the polarity of intestinal organoids can be inverted,[54]and they can even be dissociated into singlecellsand cultured as 2D monolayers[55][56]in order to make both the apical and basolateral sides of the epithelium more easily accessible. Intestinal organoids have also demonstrated therapeutic potential.[57]

In order to more accurately recapitulate the intestinein vivo,co-cultures of intestinal organoids andimmune cellshave been developed.[56]Furthermore,organ-on-a-chipmodels combine intestinal organoids with other cell types such asendothelialorimmune cellsas well asperistalticflow.[58][59]
Gastric organoid
[edit]Gastric organoids recapitulate at least partly the physiology of thestomach.Gastric organoids have been generated directly from pluripotent stem cells through the temporal manipulation of theFGF,WNT,BMP,retinoic acidandEGFsignalling pathways in three-dimensional culture conditions.[60]Gastric organoids have also been generated usingLGR5expressing stomachadult stem cells.[61]Gastric organoids have been used as model for the study ofcancer[62][63]along with human disease[60]and development.[60]For example, one study[63]investigated the underlying genetic alterations behind a patient'smetastatic tumor population,and identified that unlike the patient's primary tumor, the metastasis had both alleles of theTGFBR2gene mutated. To further assess the role of TGFBR2 in the metastasis, the investigators created organoids where TGFBR2 expression is knocked down, through which they were able to demonstrate that reduced TGFBR2 activity leads to invasion and metastasis of cancerous tumors bothin vitroandin vivo.
Lingual organoid
[edit]Lingual organoids are organoids that recapitulate, at least partly, aspects of the tongue physiology. Epithelial lingual organoids have been generated usingBMI1expressing epithelial stem cells in three-dimensional culture conditions through the manipulation ofEGF,WNT,andTGF-β.[64]This organoid culture, however, lackstaste receptors,as these cells do not arise from Bmi1 expressing epithelial stem cells.[64]Lingual taste bud organoids containing taste cells, however, have been created using theLGR5+ orCD44+ stem/progenitor cells of circumvallate (CV) papilla tissue.[65]These taste bud organoids have been successfully created both directly from isolated Lgr5- orLGR6-expressing taste stem/progenitor cells.[66]and indirectly, through the isolation, digestion, and subsequent culturing of CV tissue containing Lgr5+ or CD44+ stem/progenitor cells.[65]
Other
[edit]- Tooth organoid (TO)[67](see alsotooth regeneration)
- Thyroid organoid[68]
- Thymic organoid[69]
- Thymic organoids recapitulate at least partly the architecture andstem-cell nichefunctionality of thethymus,[70]which is a lymphoid organ where T cells mature. Thymic organoids have been generated through the seeding of thymic stromal cells in 3-dimensional culture.[70]Thymic organoids seem to successfully recapitulate the thymus' function, as co-culturing humanhematopoietic or bone marrow stem cellswith mouse thymic organoids resulted in the production ofT-cells.[70]
- Testicular organoid[71]
- Prostate organoid[72]
- Hepatic organoid.[73]A recent study showed the usefulness of the technology for identifying novel medication for the treatment ofhepatitis Eas it allows to allows to recapitulate the entire viral life cycle.[74]
- Pancreatic organoid[75][76][77][78]
- Recent advances in cell repellent microtiter plates has allowed rapid, cost-effective screening of large small molecule drug like libraries against 3D models of pancreas cancer. These models are consistent in phenotype and expression profiles with those found in the lab of Dr.David Tuveson.
- Epithelial organoid[15][79]
- Lung organoid[80]
- Kidney organoid[16][81][82][83]
- Gastruloid(embryonic organoid)[84][85][86][87]– Generates all embryonic axes and fully implements the collinearHoxgene expression patterns along the anteroposterior axis.[87]
- Blastoid(blastocyst-like organoid)[88][89][90]
- Endometrial organoid[91]
- Cardiac organoid[92]– In 2018 hollow cardiac organoids were made to beat, and to respond to stimuli to beat faster or slower.[93]
- Retinal organoid[94][95]
- Breast cancerorganoid[96]
- Colorectal cancerorganoid[97]
- Glioblastomaorganoid[98]
3D organoid models of brain cancer derived from either patient derived explants (PDX) or direct from cancer tissue is now easily achievable and affords high-throughput screening of these tumors against the current panel of approved drugs form around the world.
- Neuroendocrine tumororganoid[99]
- Myelinoid(Myelin organoid)
- Blood-brain barrier (BBB) organoid[100]
Self-assembled cell aggregates consisting of BMECs, astrocytes, and pericytes are emerging as a potential alternative to transwell and microfluidic models for certain applications. These organoides can generate many features of the BBB, such as the expression of tight junctions, molecular transporters, and drug efflux pumps, and can therefore be used to model drug transport across the BBB. Also, they can serve as a model for evaluating the interactions between the BBB and adjacent brain tissue and provide a platform for understanding the combined abilities of a new drug to overcome the BBB and its effect on brain tissue. In addition, such models are highly scalable and easier to manufacture and operate than microfluidic devices. However, they have limited ability to reconstruct the morphology and physiology of the BBB and are unable to simulate physiological flow andshear stress.
Basic research
[edit]Organoids enable to study how cells interact together in an organ, their interaction with their environment, how diseases affect them and the effect of drugs.In vitroculture makes this system easy to manipulate and facilitates their monitoring. While organs are difficult to culture because their size limits the penetration of nutrients, the small size of organoids limits this problem. On the other hand, they do not exhibit all organ features and interactions with other organs are not recapitulatedin vitro.While research onstem cellsand regulation of stemness was the first field of application of intestinal organoids,[15]they are now also used to study e.g. uptake of nutrients, drug transport and secretion ofincretinhormones.[101]This is of great relevance in the context ofmalabsorptiondiseases as well as metabolic diseases such asobesity,insulin resistance,anddiabetes.
Models of disease
[edit]Organoids provide an opportunity to create cellular models of human disease, which can be studied in the laboratory to better understand the causes of disease and identify possible treatments. The power of organoids in this regard was first shown for a genetic form ofmicrocephaly,where patient cells were used to makecerebral organoids,which were smaller and showed abnormalities in early generation of neurons.[22]In another example, the genome editing system called CRISPR was applied to human pluripotent stem cells to introduce targeted mutations in genes relevant to two different kidney diseases,polycystic kidney diseaseandfocal segmental glomerulosclerosis.[82]These CRISPR-modified pluripotent stem cells were subsequently grown into human kidney organoids, which exhibited disease-specific phenotypes. Kidney organoids from stem cells with polycystic kidney disease mutations formed large, translucent cyst structures from kidney tubules. When cultured in the absence of adherent cues (in suspension), these cysts reached sizes of 1 cm in diameter over several months.[102]Kidney organoids with mutations in a gene linked to focal segmental glomerulosclerosis developed junctional defects between podocytes, the filtering cells affected in that disease.[103]Importantly, these disease phenotypes were absent in control organoids of identical genetic background, but lacking the CRISPR mutations.[82][102][103]Comparison of these organoid phenotypes to diseased tissues from mice and humans suggested similarities to defects in early development.[102][103]
As first developed by Takahashi and Yamanaka in 2007,induced pluripotent stem cells(iPSC) can also be reprogrammed from patient skin fibroblasts.[104]These stem cells carry the exact genetic background of the patient including any genetic mutations which might contribute to the development of human disease. Differentiation of these cells into kidney organoids has been performed from patients withLowe Syndromedue toORCL1mutations.[105]This report compared kidney organoids differentiated from patient iPSC to unrelated control iPSC and demonstrated an inability of patient kidney cells to mobilise transcription factor SIX2 from thegolgi complex.[105]BecauseSIX2is a well characterised marker of nephron progenitor cells in the capmesenchyme,the authors concluded that renal disease frequently seen in Lowe Syndrome (global failure ofproximal tubulereabsorption or renalFanconi syndrome) could be related to alteration in nephron patterning arising from nephron progenitor cells lacking this importantSIX2gene expression.[105]
Other studies have used CRISPR gene editing to correct the patient's mutation in the patient iPSC cells to create anisogeniccontrol, which can be performed simultaneously with iPSC reprogramming.[106][107][108]Comparison of a patient iPSC derived organoid against an isogenic control is the current gold standard in the field as it permits isolation of the mutation of interest as the only variable within the experimental model.[109]In one such report, kidney organoids derived from iPSC of a patient withMainzer-Saldino Syndromedue tocompound heterozygousmutations inIFT140were compared to an isogenic control organoid in which anIFT140variant giving rise to a non-viable mRNA transcript was corrected by CRISPR.[107]Patient kidney organoids demonstrated abnormalciliarymorphology consistent with existing animal models which was rescued to wild type morphology in the gene corrected organoids.[107]Comparative transcriptional profiling of epithelial cells purified from patient and control organoids highlighted pathways involved incell polarity,cell-cell junctionsanddynein motorassembly, some of which had been implicated for other genotypes within the phenotypic family of renal ciliopathies.[107]Another report utilising an isogenic control demonstrated abnormalnephrinlocalisation in theglomeruliof kidney organoids generated from a patient withcongenital nephrotic syndrome.[108]
Things such as epithelial metabolism can also be modelled.[110]
Personalised medicine
[edit]Intestinal organoids grown from rectal biopsies using culture protocols established by the Clevers group have been used to modelcystic fibrosis,[111]and led to the first application of organoids for personalised treatment.[112]Cystic fibrosis is an inherited disease that is caused by gene mutations of the cystic fibrosis transmembrane conductance regulator gene that encodes an epithelial ion channel necessary for healthy epithelial surface fluids. Studies by the laboratory of Jeffrey Beekman (Wilhelmina Children's Hospital, University Medical Center Utrecht, The Netherlands) described in 2013 that stimulation of colorectal organoids with cAMP-raising agonists such as forskolin or cholera toxin induced rapid swelling of organoids in a fully CFTR dependent manner.[111]Whereas organoids from non-cystic fibrosis subjects swell in response to forskolin as a consequence of fluid transport into the organoids' lumens, this is severely reduced or absent in organoids derived from people with cystic fibrosis. Swelling could be restored by therapeutics that repair the CFTR protein (CFTR modulators), indicating that individual responses to CFTR modulating therapy could be quantitated in a preclinical laboratory setting. Schwank et al. also demonstrated that the intestinal cystic fibrosis organoid phenotype could be repaired by CRISPR-Cas9 gene editing in 2013.[113]
Follow-up studies by Dekkers et al. in 2016 revealed that quantitative differences in forskolin-induced swelling between intestinal organoids derived from people with cystic fibrosis associate with known diagnostic and prognostic markers such as CFTR gene mutations or in vivo biomarkers of CFTR function.[112]In addition, the authors demonstrated that CFTR modulator responses in intestinal organoids with specific CFTR mutations correlated with published clinical trial data of these treatments. This led to preclinical studies where organoids from patients with extremely rare CFTR mutations for who no treatment was registered were found to respond strongly to a clinically available CFTR modulator. The suggested clinical benefit of treatment for these subjects based on the preclinical organoid test was subsequently confirmed upon clinical introduction of treatment by members of the clinical CF center under supervision of Kors van der Ent (Department of Paediatric Pulmonology, Wilhelmina Children's Hospital, University Medical Center Utrecht, The Netherlands). These studies show for the first time that organoids can be used for the individual tailoring of therapy orpersonalised medicine.
Organoid transplants
[edit]The first successfultransplantationof an organoid into a human, a patient withulcerative colitiswhose cells were used for the organoid, was carried out in 2022.[114][115]
As a model for developmental biology
[edit]Organoids offer researchers an exceptional model to studydevelopmental biology.[116]Since the identification ofpluripotent stem cells,there have been great advancements indirecting pluripotent stem cells fatein vitrousing 2D cultures.[116]These advancements in PSC fate direction, coupled with the advancements in 3D culturing techniques allowed for the creation of organoids that recapitulate the properties of various specific subregions of a multitude of organs.[116]The use of these organoids has thus greatly contributed to expanding our understanding of the processes oforganogenesis,and the field of developmental biology.[116]Incentral nervous systemdevelopment, for example, organoids have contributed to our understanding of the physical forces that underlie retinal cup formation.[116][117]More recent work has extended cortical organoid growth periods extensively and at nearly a year under specific differentiation conditions, the organoids persist and have some features of human fetal development stages.[118]
See also
[edit]References
[edit]- ^Zhao Z, Chen X, Dowbaj AM, Sljukic A, Bratlie K, Lin L, et al. (2022)."Organoids".Nature Reviews. Methods Primers.2.doi:10.1038/s43586-022-00174-y.PMC10270325.PMID37325195.
- ^Grens K (December 24, 2013)."2013's Big Advances in Science".The Scientist.Retrieved26 December2013.
- ^Mullard A (March 2023). "Mini-organs attract big pharma".Nature Reviews. Drug Discovery.22(3): 175–176.doi:10.1038/d41573-023-00030-y.PMID36797431.
- ^abcdefghijklmnopqLancaster MA, Knoblich JA (July 2014). "Organogenesis in a dish: modeling development and disease using organoid technologies".Science.345(6194): 1247125.doi:10.1126/science.1247125.PMID25035496.S2CID16105729.
- ^Wilson HV (June 1907)."A New Method by Which Sponges May Be Artificially Reared".Science.25(649): 912–915.Bibcode:1907Sci....25..912W.doi:10.1126/science.25.649.912.PMID17842577.
- ^Holtfreter J (1944). "Experimental studies on the development of the pronephros".Rev. Can. Biol.3:220–250.
- ^Weiss P, Taylor AC (September 1960)."Reconstitution of Complete Organs From Single-Cell Suspensions of Chick Embryos in Advanced Stages of Differentiation".Proceedings of the National Academy of Sciences of the United States of America.46(9): 1177–1185.Bibcode:1960PNAS...46.1177W.doi:10.1073/pnas.46.9.1177.PMC223021.PMID16590731.
- ^Rheinwald JG, Green H (November 1975). "Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma".Cell.6(3): 317–330.doi:10.1016/0092-8674(75)90183-x.PMID1052770.S2CID28185779.
- ^abYi SA, Zhang Y, Rathnam C, Pongkulapa T, Lee KB (November 2021)."Bioengineering Approaches for the Advanced Organoid Research".Advanced Materials.33(45): e2007949.Bibcode:2021AdM....3307949Y.doi:10.1002/adma.202007949.PMC8682947.PMID34561899.
- ^Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ (January 1987)."Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells".Proceedings of the National Academy of Sciences of the United States of America.84(1): 136–140.Bibcode:1987PNAS...84..136L.doi:10.1073/pnas.84.1.136.PMC304157.PMID3467345.
- ^Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ (February 1989)."Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane".Development.105(2): 223–235.doi:10.1242/dev.105.2.223.PMC2948482.PMID2806122.
- ^Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, et al. (May 1995)."Laminin mediates tissue-specific gene expression in mammary epithelia".The Journal of Cell Biology.129(3): 591–603.doi:10.1083/jcb.129.3.591.PMC2120432.PMID7730398.
- ^Nahmias Y, Schwartz RE, Hu WS, Verfaillie CM, Odde DJ (June 2006). "Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro".Tissue Engineering.12(6): 1627–1638.doi:10.1089/ten.2006.12.1627.PMID16846358.
- ^Yong E(August 28, 2013)."Lab-Grown Model Brains".The Scientist.Retrieved26 December2013.
- ^abcdeSato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. (May 2009). "Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche".Nature.459(7244): 262–265.Bibcode:2009Natur.459..262S.doi:10.1038/nature07935.PMID19329995.S2CID4373784.
- ^abUnbekandt M, Davies JA (March 2010)."Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues".Kidney International.77(5): 407–416.doi:10.1038/ki.2009.482.PMID20016472.
- ^Peng H, Poovaiah N, Forrester M, Cochran E, Wang Q (December 2014). "Ex Vivo Culture of Primary Intestinal Stem Cells in Collagen Gels and Foams".ACS Biomaterials Science & Engineering.1(1): 37–42.doi:10.1021/ab500041d.PMID33435081.
- ^Peng H, Wang C, Xu X, Yu C, Wang Q (January 2015). "An intestinal Trojan horse for gene delivery".Nanoscale.7(10): 4354–4360.doi:10.1039/c4nr06377e.PMID25619169.
- ^Lawrence ML, Chang CH, Davies JA (March 2015)."Transport of organic anions and cations in murine embryonic kidney development and in serially-reaggregated engineered kidneys".Scientific Reports.5:9092.Bibcode:2015NatSR...5E9092L.doi:10.1038/srep09092.PMC4357899.PMID25766625.
- ^Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, et al. (November 2012)."In vivo maturation of functional renal organoids formed from embryonic cell suspensions".Journal of the American Society of Nephrology.23(11): 1857–1868.doi:10.1681/ASN.2012050505.PMC3482737.PMID23085631.
- ^Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, et al. (March 2012). "Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5⁺ stem cell".Nature Medicine.18(4): 618–623.doi:10.1038/nm.2695.PMID22406745.
- ^abcLancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. (September 2013)."Cerebral organoids model human brain development and microcephaly".Nature.501(7467): 373–379.Bibcode:2013Natur.501..373L.doi:10.1038/nature12517.PMC3817409.PMID23995685.
- ^Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, et al. (February 2013)."In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration".Nature.494(7436): 247–250.doi:10.1038/nature11826.PMC3634804.PMID23354049.
- ^Shkumatov A, Baek K, Kong H (April 2014)."Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies".PLOS ONE.9(4): e94764.Bibcode:2014PLoSO...994764S.doi:10.1371/journal.pone.0094764.PMC3986240.PMID24732893.
{{cite journal}}:CS1 maint: year (link) - ^Murry CE, Keller G (February 2008)."Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development".Cell.132(4): 661–680.doi:10.1016/j.cell.2008.02.008.PMID18295582.
- ^Choudhury D, Ashok A, Naing MW (March 2020). "Commercialization of Organoids".Trends in Molecular Medicine.26(3): 245–249.doi:10.1016/j.molmed.2019.12.002.PMID31982341.S2CID210922708.
- ^Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ (January 1987)."Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells".Proceedings of the National Academy of Sciences of the United States of America.84(1): 136–140.Bibcode:1987PNAS...84..136L.doi:10.1073/pnas.84.1.136.PMC304157.PMID3467345.
- ^abcPastuła A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, et al. (2016)."Three-Dimensional Gastrointestinal Organoid Culture in Combination with Nerves or Fibroblasts: A Method to Characterize the Gastrointestinal Stem Cell Niche".Stem Cells International.2016:3710836.doi:10.1155/2016/3710836.PMC4677245.PMID26697073.
- ^Takahashi K, Yamanaka S (August 2006). "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors".Cell.126(4): 663–676.doi:10.1016/j.cell.2006.07.024.hdl:2433/159777.PMID16904174.
- ^Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (November 2013)."Genome engineering using the CRISPR-Cas9 system".Nature Protocols.8(11): 2281–2308.doi:10.1038/nprot.2013.143.PMC3969860.PMID24157548.
- ^Lavazza A, Massimini M (September 2018)."Cerebral organoids: ethical issues and consciousness assessment".Journal of Medical Ethics.44(9): 606–610.doi:10.1136/medethics-2017-104555.PMID29491041.
- ^Scully RP (6 July 2019)."Miniature brains grown in the lab have human-like neural activity".New Scientist.No. 3237.
- ^Sample I (21 October 2019)."Scientists 'may have crossed ethical line' in growing human brains".The Guardian.p. 15.
- ^Cai H, Ao Z, Tian C, et al. (2023). "Brain organoid reservoir computing for artificial intelligence".Nat Electron.6(12): 1032–1039.doi:10.1038/s41928-023-01069-w.
- ^Smirnova L, Caffo BS, Gracias DH, Huang Q, Morales Pantoja IE, Tang B, et al. (2023)."Organoid intelligence (OI): the new frontier in biocomputing and intelligence-in-a-dish".Front Sci.1:1017235.doi:10.3389/fsci.2023.1017235.
- ^Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. (November 2011)."Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium".Gastroenterology.141(5): 1762–1772.doi:10.1053/j.gastro.2011.07.050.PMID21889923.
- ^Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, et al. (September 2011). "Isolation and in vitro expansion of human colonic stem cells".Nature Medicine.17(10): 1225–1227.doi:10.1038/nm.2470.PMID21892181.S2CID205388154.
- ^Cai T, Qi Y, Jergens A, Wannemuehler M, Barrett TA, Wang Q (2018)."Effects of six common dietary nutrients on murine intestinal organoid growth".PLOS ONE.13(2): e0191517.doi:10.1371/journal.pone.0191517.PMC5794098.PMID29389993.
- ^Qi Y, Lohman J, Bratlie KM, Peroutka-Bigus N, Bellaire B, Wannemuehler M, et al. (September 2019)."Vitamin C and B3as new biomaterials to alter intestinal stem cells ".Journal of Biomedical Materials Research. Part A.107(9): 1886–1897.doi:10.1002/jbm.a.36715.PMC6626554.PMID31071241.
- ^Davoudi Z, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Barrett TA, Narasimhan B, et al. (April 2018)."Intestinal organoids containing poly(lactic-co-glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases".Journal of Biomedical Materials Research. Part A.106(4): 876–886.doi:10.1002/jbm.a.36305.PMC5826879.PMID29226615.
- ^Davoudi Z, Peroutka-Bigus N, Bellaire B, Jergens A, Wannemuehler M, Wang Q (May 2021)."Gut Organoid as a New Platform to Study Alginate and Chitosan Mediated PLGA Nanoparticles for Drug Delivery".Marine Drugs.19(5): 282.doi:10.3390/md19050282.PMC8161322.PMID34065505.
- ^Qi Y, Shi E, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Jergens A, et al. (May 2018). "Ex VivoStudy of Telluride Nanowires in Minigut ".Journal of Biomedical Nanotechnology.14(5): 978–986.doi:10.1166/jbn.2018.2578.PMID29883567.
- ^Reding B, Carter P, Qi Y, Li Z, Wu Y, Wannemuehler M, et al. (April 2021)."Manipulate intestinal organoids with niobium carbide nanosheets".Journal of Biomedical Materials Research. Part A.109(4): 479–487.doi:10.1002/jbm.a.37032.PMID32506610.
- ^Zietek T, Giesbertz P, Ewers M, Reichart F, Weinmüller M, Urbauer E, et al. (2020)."Organoids to Study Intestinal Nutrient Transport, Drug Uptake and Metabolism - Update to the Human Model and Expansion of Applications".Frontiers in Bioengineering and Biotechnology.8:577656.doi:10.3389/fbioe.2020.577656.PMC7516017.PMID33015026.
- ^Zietek T, Rath E, Haller D, Daniel H (November 2015)."Intestinal organoids for assessing nutrient transport, sensing and incretin secretion".Scientific Reports.5(1): 16831.Bibcode:2015NatSR...516831Z.doi:10.1038/srep16831.PMC4652176.PMID26582215.
- ^Rahmani S, Breyner NM, Su HM, Verdu EF, Didar TF (February 2019). "Intestinal organoids: A new paradigm for engineering intestinal epithelium in vitro".Biomaterials.194:195–214.doi:10.1016/j.biomaterials.2018.12.006.PMID30612006.S2CID58603850.
- ^Sun L, Rollins D, Qi Y, Fredericks J, Mansell TJ, Jergens A, et al. (December 2020)."TNFα regulates intestinal organoids from mice with both defined and conventional microbiota".International Journal of Biological Macromolecules.164:548–556.doi:10.1016/j.ijbiomac.2020.07.176.PMC7657954.PMID32693143.
- ^Tong T, Qi Y, Rollins D, Bussiere LD, Dhar D, Miller CL, et al. (December 2023)."Rational design of oral drugs targeting mucosa delivery with gut organoid platforms".Bioactive Materials.30:116–128.doi:10.1016/j.bioactmat.2023.07.014.PMC10406959.PMID37560199.
- ^Davoudi Z, Atherly T, Borcherding DC, Jergens AE, Wannemuehler M, Barrett TA, et al. (December 2023). "Study Transportation of Drugs within Newly Established Murine Colon Organoid Systems".Advanced Biology.7(12): e2300103.doi:10.1002/adbi.202300103.PMC10840714.PMID37607116.
- ^Lindemans C, Mertelsmann A, Dudakov JA, Velardi E, Hua G, O'Connor M, et al. (2014)."IL-22 Administration Protects Intestinal Stem Cells from Gvhd".Biology of Blood and Marrow Transplantation.20(2): S53–S54.doi:10.1016/j.bbmt.2013.12.056.
- ^Zhang YG, Wu S, Xia Y, Sun J (September 2014)."Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions".Physiological Reports.2(9): e12147.doi:10.14814/phy2.12147.PMC4270227.PMID25214524.
- ^Geiser P, Di Martino ML, Samperio Ventayol P, Eriksson J, Sima E, Al-Saffar AK, et al. (January 2021). Sperandio V (ed.)."Salmonella enterica Serovar Typhimurium Exploits Cycling through Epithelial Cells To Colonize Human and Murine Enteroids".mBio.12(1).doi:10.1128/mBio.02684-20.PMC7844539.PMID33436434.
- ^Dutta D, Heo I, O'Connor R (September 2019). "Studying Cryptosporidium Infection in 3D Tissue-derived Human Organoid Culture Systems by Microinjection".Journal of Visualized Experiments(151): 59610.doi:10.3791/59610.PMID31566619.S2CID203377662.
- ^Co JY, Margalef-Català M, Li X, Mah AT, Kuo CJ, Monack DM, et al. (February 2019)."Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions".Cell Reports.26(9): 2509–2520.e4.doi:10.1016/j.celrep.2019.01.108.PMC6391775.PMID30811997.
- ^Tong T, Qi Y, Bussiere LD, Wannemuehler M, Miller CL, Wang Q, et al. (August 2020)."Transport of artificial virus-like nanocarriers through intestinal monolayers via microfold cells".Nanoscale.12(30): 16339–16347.doi:10.1039/d0nr03680c.PMID32725029.
- ^abNoel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, et al. (March 2017)."A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions".Scientific Reports.7(1): 45270.Bibcode:2017NatSR...745270N.doi:10.1038/srep45270.PMC5366908.PMID28345602.
- ^Bouchi R, Foo KS, Hua H, Tsuchiya K, Ohmura Y, Sandoval PR, et al. (June 2014)."FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures".Nature Communications.5:4242.Bibcode:2014NatCo...5.4242B.doi:10.1038/ncomms5242.PMC4083475.PMID24979718.
- ^Sontheimer-Phelps A, Chou DB, Tovaglieri A, Ferrante TC, Duckworth T, Fadel C, et al. (2020)."Human Colon-on-a-Chip Enables Continuous In Vitro Analysis of Colon Mucus Layer Accumulation and Physiology".Cellular and Molecular Gastroenterology and Hepatology.9(3): 507–526.doi:10.1016/j.jcmgh.2019.11.008.PMC7036549.PMID31778828.
- ^Grassart A, Malardé V, Gobaa S, Sartori-Rupp A, Kerns J, Karalis K, et al. (September 2019)."Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection".Cell Host & Microbe.26(3): 435–444.e4.doi:10.1016/j.chom.2019.08.007.PMID31492657.S2CID201868491.
- ^abcMcCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, et al. (December 2014)."Modelling human development and disease in pluripotent stem-cell-derived gastric organoids".Nature.516(7531): 400–404.Bibcode:2014Natur.516..400M.doi:10.1038/nature13863.PMC4270898.PMID25363776.
- ^Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, et al. (January 2010)."Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro".Cell Stem Cell.6(1): 25–36.doi:10.1016/j.stem.2009.11.013.PMID20085740.
- ^Li X, Nadauld L, Ootani A, Corney DC, Pai RK, Gevaert O, et al. (July 2014)."Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture".Nature Medicine.20(7): 769–777.doi:10.1038/nm.3585.PMC4087144.PMID24859528.
- ^abNadauld LD, Garcia S, Natsoulis G, Bell JM, Miotke L, Hopmans ES, et al. (August 2014)."Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer".Genome Biology.15(8): 428.doi:10.1186/s13059-014-0428-9.PMC4145231.PMID25315765.
- ^abHisha H, Tanaka T, Kanno S, Tokuyama Y, Komai Y, Ohe S, et al. (November 2013)."Establishment of a novel lingual organoid culture system: generation of organoids having mature keratinized epithelium from adult epithelial stem cells".Scientific Reports.3:3224.Bibcode:2013NatSR...3E3224H.doi:10.1038/srep03224.PMC3828633.PMID24232854.
- ^abAihara E, Mahe MM, Schumacher MA, Matthis AL, Feng R, Ren W, et al. (November 2015)."Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid".Scientific Reports.5:17185.Bibcode:2015NatSR...517185A.doi:10.1038/srep17185.PMC4665766.PMID26597788.
- ^Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, et al. (November 2014)."Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo".Proceedings of the National Academy of Sciences of the United States of America.111(46): 16401–16406.Bibcode:2014PNAS..11116401R.doi:10.1073/pnas.1409064111.PMC4246268.PMID25368147.
- ^Hermans F, Hasevoets S, Vankelecom H, Bronckaers A, Lambrichts I (March 2024)."From Pluripotent Stem Cells to Organoids and Bioprinting: Recent Advances in Dental Epithelium and Ameloblast Models to Study Tooth Biology and Regeneration".Stem Cell Reviews and Reports.doi:10.1007/s12015-024-10702-w.PMC11222197.PMID38498295.
- ^Martin A, Barbesino G, Davies TF (1999). "T-cell receptors and autoimmune thyroid disease--signposts for T-cell-antigen driven diseases".International Reviews of Immunology.18(1–2): 111–140.doi:10.3109/08830189909043021.PMID10614741.
- ^Bredenkamp N, Ulyanchenko S, O'Neill KE, Manley NR, Vaidya HJ, Blackburn CC (September 2014)."An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts".Nature Cell Biology.16(9): 902–908.doi:10.1038/ncb3023.PMC4153409.PMID25150981.
- ^abcVianello F, Poznansky MC (2007). "Generation of a tissue-engineered thymic organoid".Immunological Tolerance.Methods in Molecular Biology. Vol. 380. pp. 163–70.doi:10.1007/978-1-59745-395-0_9.ISBN978-1-59745-395-0.PMID17876092.
- ^Sakib S, Uchida A, Valenzuela-Leon P, Yu Y, Valli-Pulaski H, Orwig K, et al. (June 2019)."Formation of organotypic testicular organoids in microwell culture†".Biology of Reproduction.100(6): 1648–1660.doi:10.1093/biolre/ioz053.PMC7302515.PMID30927418.
- ^Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, Chen Y, et al. (February 2016)."Organoid culture systems for prostate epithelial and cancer tissue".Nature Protocols.11(2): 347–358.doi:10.1038/nprot.2016.006.PMC4793718.PMID26797458.
- ^Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. (January 2015)."Long-term culture of genome-stable bipotent stem cells from adult human liver".Cell.160(1–2): 299–312.doi:10.1016/j.cell.2014.11.050.PMC4313365.PMID25533785.
- ^Li P, Li Y, Wang Y, Liu J, Lavrijsen M, Li Y, et al. (January 2022)."Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids".Science Advances.8(3): eabj5908.Bibcode:2022SciA....8.5908L.doi:10.1126/sciadv.abj5908.hdl:11250/3047921.PMC8769558.PMID35044825.S2CID246069868.
- ^Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, et al. (October 2013)."Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis".The EMBO Journal.32(20): 2708–2721.doi:10.1038/emboj.2013.204.PMC3801438.PMID24045232.
- ^Hou S, Tiriac H, Sridharan BP, Scampavia L, Madoux F, Seldin J, et al. (July 2018)."Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening".SLAS Discovery.23(6): 574–584.doi:10.1177/2472555218766842.PMC6013403.PMID29673279.
- ^Wolff RA, Wang-Gillam A, Alvarez H, Tiriac H, Engle D, Hou S, et al. (March 2018)."Dynamic changes during the treatment of pancreatic cancer".Oncotarget.9(19): 14764–14790.doi:10.18632/oncotarget.24483.PMC5871077.PMID29599906.
- ^Below CR, Kelly J, Brown A, Humphries JD, Hutton C, Xu J, et al. (January 2022)."A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids".Nature Materials.21(1): 110–119.doi:10.1038/s41563-021-01085-1.PMC7612137.PMID34518665.
- ^Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. (October 2007). "Identification of stem cells in small intestine and colon by marker gene Lgr5".Nature.449(7165): 1003–1007.Bibcode:2007Natur.449.1003B.doi:10.1038/nature06196.PMID17934449.S2CID4349637.
- ^Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. (January 2014)."Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis".Cell.156(3): 440–455.doi:10.1016/j.cell.2013.12.039.PMC3951122.PMID24485453.
- ^Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. (October 2015). "Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis".Nature.526(7574): 564–568.Bibcode:2015Natur.526..564T.doi:10.1038/nature15695.PMID26444236.S2CID4443766.
- ^abcFreedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, et al. (October 2015)."Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids".Nature Communications.6:8715.Bibcode:2015NatCo...6.8715F.doi:10.1038/ncomms9715.PMC4620584.PMID26493500.
- ^Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV (November 2015)."Nephron organoids derived from human pluripotent stem cells model kidney development and injury".Nature Biotechnology.33(11): 1193–1200.doi:10.1038/nbt.3392.PMC4747858.PMID26458176.
- ^van den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, et al. (November 2014)."Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells".Development.141(22): 4231–4242.doi:10.1242/dev.113001.PMC4302915.PMID25371360.
- ^Turner DA, Baillie-Johnson P, Martinez Arias A (February 2016)."Organoids and the genetically encoded self-assembly of embryonic stem cells".BioEssays.38(2): 181–191.doi:10.1002/bies.201500111.PMC4737349.PMID26666846.
- ^Turner DA, Girgin M, Alonso-Crisostomo L, Trivedi V, Baillie-Johnson P, Glodowski CR, et al. (November 2017)."Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids".Development.144(21): 3894–3906.doi:10.1242/dev.150391.PMC5702072.PMID28951435.
- ^abBeccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, et al. (October 2018). "Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids".Nature.562(7726): 272–276.Bibcode:2018Natur.562..272B.doi:10.1038/s41586-018-0578-0.PMID30283134.S2CID52915553.
- ^Rivron N (27 June 2018)."Blastoid: The backstory of the formation of blastocyst-like structure solely from stem cells".The Node.The Company of Biologists Ltd.
- ^"Blastoid".Nicolas Rivron Lab.Netherlands.
- ^Rivron NC, Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivié J, et al. (May 2018). "Blastocyst-like structures generated solely from stem cells".Nature.557(7703): 106–111.Bibcode:2018Natur.557..106R.doi:10.1038/s41586-018-0051-0.PMID29720634.S2CID13749109.
- ^Rawlings TM, Makwana K, Tryfonos M, Lucas ES (July 2021)."Organoids to model the endometrium: implantation and beyond".Reproduction & Fertility.2(3): R85–R101.doi:10.1530/RAF-21-0023.PMC8801025.PMID35118399.
- ^Lee EJ, Kim DE, Azeloglu EU, Costa KD (February 2008). "Engineered cardiac organoid chambers: toward a functional biological model ventricle".Tissue Engineering. Part A.14(2): 215–225.doi:10.1089/tea.2007.0351.PMID18333774.
- ^Molteni M (2018-06-27)."These Beating Mini-Hearts Could Save Big Bucks—And Maybe Lives".WIRED.Retrieved2018-06-30.
- ^Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, et al. (July 2016)."cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness".Scientific Reports.6:30742.Bibcode:2016NatSR...630742W.doi:10.1038/srep30742.PMC4965859.PMID27471043.
- ^Zilova L, Weinhardt V, Tavhelidse T, Schlagheck C, Thumberger T, Wittbrodt J (July 2021). Arias AM, Stainier DY (eds.)."Fish primary embryonic pluripotent cells assemble into retinal tissue mirroring in vivo early eye development".eLife.10:e66998.doi:10.7554/eLife.66998.PMC8275126.PMID34252023.
- ^Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. (January 2018)."A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity".Cell.172(1–2): 373–386.e10.doi:10.1016/j.cell.2017.11.010.PMID29224780.
- ^van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. (May 2015)."Prospective derivation of a living organoid biobank of colorectal cancer patients".Cell.161(4): 933–945.doi:10.1016/j.cell.2015.03.053.PMC6428276.PMID25957691.
- ^Quereda V, Hou S, Madoux F, Scampavia L, Spicer TP, Duckett D (September 2018)."A Cytotoxic Three-Dimensional-Spheroid, High-Throughput Assay Using Patient-Derived Glioma Stem Cells".SLAS Discovery.23(8): 842–849.doi:10.1177/2472555218775055.PMC6102052.PMID29750582.
- ^Dayton TL, Alcala N, Moonen L, den Hartigh L, Geurts V, Mangiante L, et al. (December 2023)."Druggable growth dependencies and tumor evolution analysis in patient-derived organoids of neuroendocrine neoplasms from multiple body sites".Cancer Cell.41(12): 2083–2099.e9.doi:10.1016/j.ccell.2023.11.007.PMID38086335.
- ^Zidarič T, Gradišnik L, Velnar T (September 2022)."Astrocytes and human artificial blood-brain barrier models".Bosnian Journal of Basic Medical Sciences.22(5): 651–672.doi:10.17305/bjbms.2021.6943.PMC9519155.PMID35366791.
- ^Zietek T, Rath E, Haller D, Daniel H (November 2015)."Intestinal organoids for assessing nutrient transport, sensing and incretin secretion".Scientific Reports.5:16831.Bibcode:2015NatSR...516831Z.doi:10.1038/srep16831.PMC4652176.PMID26582215.
- ^abcCruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, et al. (November 2017)."Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease".Nature Materials.16(11): 1112–1119.Bibcode:2017NatMa..16.1112C.doi:10.1038/nmat4994.PMC5936694.PMID28967916.
- ^abcKim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, et al. (December 2017)."Gene-Edited Human Kidney Organoids Reveal Mechanisms of Disease in Podocyte Development".Stem Cells.35(12): 2366–2378.doi:10.1002/stem.2707.PMC5742857.PMID28905451.
- ^Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. (November 2007). "Induction of pluripotent stem cells from adult human fibroblasts by defined factors".Cell.131(5): 861–872.doi:10.1016/j.cell.2007.11.019.hdl:2433/49782.PMID18035408.S2CID8531539.
- ^abcHsieh WC, Ramadesikan S, Fekete D, Aguilar RC (2018-02-14)."Kidney-differentiated cells derived from Lowe Syndrome patient's iPSCs show ciliogenesis defects and Six2 retention at the Golgi complex".PLOS ONE.13(2): e0192635.Bibcode:2018PLoSO..1392635H.doi:10.1371/journal.pone.0192635.PMC5812626.PMID29444177.
- ^Howden SE, Thomson JA, Little MH (May 2018)."Simultaneous reprogramming and gene editing of human fibroblasts".Nature Protocols.13(5): 875–898.doi:10.1038/nprot.2018.007.PMC5997775.PMID29622803.
- ^abcdForbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, et al. (May 2018)."Patient-iPSC-Derived Kidney Organoids Show Functional Validation of a Ciliopathic Renal Phenotype and Reveal Underlying Pathogenetic Mechanisms".American Journal of Human Genetics.102(5): 816–831.doi:10.1016/j.ajhg.2018.03.014.PMC5986969.PMID29706353.
- ^abTanigawa S, Islam M, Sharmin S, Naganuma H, Yoshimura Y, Haque F, et al. (September 2018)."Organoids from Nephrotic Disease-Derived iPSCs Identify Impaired NEPHRIN Localization and Slit Diaphragm Formation in Kidney Podocytes".Stem Cell Reports.11(3): 727–740.doi:10.1016/j.stemcr.2018.08.003.PMC6135868.PMID30174315.
- ^Engle SJ, Blaha L, Kleiman RJ (November 2018)."Best Practices for Translational Disease Modeling Using Human iPSC-Derived Neurons".Neuron.100(4): 783–797.doi:10.1016/j.neuron.2018.10.033.PMID30465765.
- ^Rath E, Chamaillard M (eds.)."Metabolites".Special Issue of the Metabolites Journal.Retrieved2022-10-16.
- ^abDekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, et al. (July 2013). "A functional CFTR assay using primary cystic fibrosis intestinal organoids".Nature Medicine.19(7): 939–945.doi:10.1038/nm.3201.PMID23727931.S2CID5369669.
- ^abDekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, et al. (June 2016). "Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis".Science Translational Medicine.8(344): 344ra84.doi:10.1126/scitranslmed.aad8278.PMID27334259.S2CID19462535.
- ^Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, et al. (December 2013)."Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients".Cell Stem Cell.13(6): 653–658.doi:10.1016/j.stem.2013.11.002.PMID24315439.
- ^"World's first mini organ transportation to a patient with ulcerative colitis".Tokyo Medical and Dental Universityvia medicalxpress.Retrieved18 September2022.
- ^Watanabe S, Kobayashi S, Ogasawara N, Okamoto R, Nakamura T, Watanabe M, et al. (March 2022). "Transplantation of intestinal organoids into a mouse model of colitis".Nature Protocols.17(3): 649–671.doi:10.1038/s41596-021-00658-3.PMID35110738.S2CID246488596.
- ^abcdeAder M, Tanaka EM (December 2014). "Modeling human development in 3D culture".Current Opinion in Cell Biology.31:23–28.doi:10.1016/j.ceb.2014.06.013.PMID25033469.
- ^Martinez-Morales JR, Cavodeassi F, Bovolenta P (2017)."Coordinated Morphogenetic Mechanisms Shape the Vertebrate Eye".Frontiers in Neuroscience.11:721.doi:10.3389/fnins.2017.00721.PMC5742352.PMID29326547.
- ^Gordon A, Yoon SJ, Tran SS, Makinson CD, Park JY, Andersen J, et al. (March 2021)."Long-term maturation of human cortical organoids matches key early postnatal transitions".Nature Neuroscience.24(3): 331–342.doi:10.1038/s41593-021-00802-y.PMC8109149.PMID33619405.
Further reading
[edit]- Chi KR (September 2015)."Orchestrating Organoids. A guide to crafting tissues in a dish that reprise in vivo organs".The Scientist.
- Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, et al. (May 2015)."Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation".Cell Stem Cell.16(5): 556–565.doi:10.1016/j.stem.2015.03.004.PMID25891906.
- Turner DA, Baillie-Johnson P, Martinez Arias A (February 2016)."Organoids and the genetically encoded self-assembly of embryonic stem cells".BioEssays.38(2): 181–191.doi:10.1002/bies.201500111.PMC4737349.PMID26666846.
- Willyard C (July 2015)."The boom in mini stomachs, brains, breasts, kidneys and more".Nature.523(7562): 520–522.Bibcode:2015Natur.523..520W.doi:10.1038/523520a.PMID26223610.
External links
[edit]- "Organoid-based regenerative medicine".Longevity Wiki.30 March 2024.
