Phosphorus pentoxide
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Tetraphosphorus decaoxide
Tricyclo[3.3.1.13,7]tetraphosphoxane 1,3,5,7-tetraoxide | |||
| Systematic IUPAC name
2,4,6,8,9,10-Hexaoxa-1λ5,3λ5,5λ5,7λ5-tetraphosphatricyclo[3.3.1.13,7]decane 1,3,5,7-tetraoxide | |||
| Other names
Diphosphorus pentoxide
Phosphorus(V) oxide Phosphoric anhydride Tetraphosphorus decaoxide Tetraphosphorus decoxide | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.013.852 | ||
PubChemCID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| P4O10 | |||
| Molar mass | 283.9 g mol−1 | ||
| Appearance | White powder Verydeliquescent | ||
| Odor | Odorless | ||
| Density | 2.39 g/cm3 | ||
| Melting point | 340 °C (644 °F; 613 K) | ||
| Boiling point | 360 °C (sublimes) | ||
| exothermichydrolysis | |||
| Vapor pressure | 1 mmHg @ 385 °C (stable form) | ||
| Hazards | |||
| Occupational safety and health(OHS/OSH): | |||
Main hazards
|
reactswith water, strongdehydrating agent,corrosive | ||
| GHSlabelling: | |||

| |||
| Danger | |||
| H314 | |||
| P280,P301+P330+P331,P303+P361+P353,P305+P351+P338,P310 | |||
| NFPA 704(fire diamond) | |||
| Safety data sheet(SDS) | MSDS | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
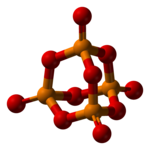
Phosphorus pentoxideis achemical compoundwith molecular formulaP4O10(with its common name derived from itsempirical formula,P2O5). This white crystalline solid is theanhydrideofphosphoric acid.It is a powerfuldesiccantanddehydrating agent.
Structure
[edit]Phosphorus pentoxide crystallizes in at least four forms orpolymorphs.The most familiar one, a metastable form[1](shown in the figure), comprises molecules of P4O10.Weakvan der Waals forceshold these molecules together in a hexagonal lattice (However, in spite of the high symmetry of the molecules, the crystal packing is not a close packing[2]). The structure of the P4O10cage is reminiscent ofadamantanewithTdsymmetry point group.[3]It is closely related to the corresponding anhydride ofphosphorous acid,P4O6.The latter lacks terminal oxo groups. Its density is 2.30 g/cm3.It boils at 423 °C under atmospheric pressure; if heated more rapidly it can sublimate. This form can be made by condensing the vapor of phosphorus pentoxide rapidly, and the result is an extremely hygroscopic solid.[4]
The other polymorphs are polymeric, but in each case the phosphorus atoms are bound by a tetrahedron of oxygen atoms, one of which forms a terminal P=O bond involving the donation of the terminal oxygen p-orbital electrons to the antibonding phosphorus-oxygen single bonds. The macromolecular form can be made by heating the compound in a sealed tube for several hours, and maintaining the melt at a high temperature before cooling the melt to the solid.[4]The metastable orthorhombic "O" -form (density 2.72 g/cm3,melting point562 °C) adopts a layered structure consisting of interconnected P6O6rings, not unlike the structure adopted by certain polysilicates.The stable form is a higher density phase, also orthorhombic, the so-called O' form. It consists of a 3-dimensional framework, density 3.5 g/cm3.[1][5]The remaining polymorph is aglassor amorphous form; it can be made by fusing any of the others.
 |
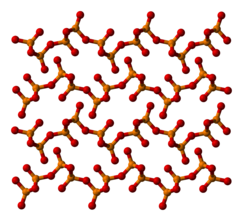 |
| Part of an o′-(P2O5)∞layer | o′-(P2O5)∞layers stacking |
Preparation
[edit]P4O10is prepared by burningwhite phosphoruswith a sufficient supply of oxygen:[6]
- P4+ 5 O2→ P4O10
The dehydration ofphosphoric acidto give phosphorus pentoxide is not possible, as on heating it forms variouspolyphosphatesbut will not dehydrate sufficiently to form P4O10.
Applications
[edit]Phosphorus pentoxide is a potentdehydratingagent as indicated by the exothermic nature of its hydrolysis producingphosphoric acid:
- P4O10+ 6 H2O → 4 H3PO4(–177kJ)
However, its utility for drying is limited somewhat by its tendency to form a protective viscous coating that inhibits further dehydration by unspent material. A granular form of P4O10is used indesiccators.
Consistent with its strong desiccating power, P4O10is used inorganic synthesisfor dehydration. The most important application is for the conversion of primaryamidesintonitriles:[7]
- P4O10+ RC(O)NH2→ P4O9(OH)2+ RCN
The indicated coproduct P4O9(OH)2is an idealized formula for undefined products resulting from the hydration of P4O10.
Alternatively, when combined with acarboxylic acid,the result is the correspondinganhydride:[8]
- P4O10+ RCO2H → P4O9(OH)2+ [RC(O)]2O
The "Onodera reagent", a solution of P4O10inDMSO,is employed for the oxidation ofalcohols.[9]This reaction is reminiscent of theSwern oxidation.
The desiccating power of P4O10is strong enough to convert many mineral acids to their anhydrides. Examples:HNO3is converted toN2O5;H2SO4is converted toSO3;HClO4is converted toCl2O7;CF3SO3His converted to(CF3)2S2O5.
As a proxy measurement
[edit]P2O5content is often used by industry as proxy value for all the phosphorus oxides in a material. For example, fertilizer grade phosphoric acid can also contain variousrelated phosphorous compoundswhich are also of use. All these compounds are described collectively in terms of 'P2O5content' to allow convenient comparison of the phosphorous content of different products. Despite this, phosphorus pentoxide is not actually present in most samples as it is not stable in aqueous solutions.
Related phosphorus oxides
[edit]Between the commercially importantP4O6and P4O10,phosphorus oxides are known with intermediate structures.[10]

On observation it will be seen that double bonded oxygen inat 1,2 position or 1,3 position are identical and both positions have same steric hindrance. Cycle 12341 and ABCDA are identical.
Hazards
[edit]Phosphorus pentoxide itself is not flammable. Just likesulfur trioxide,it reacts vigorously with water and water-containing substances like wood or cotton, liberates much heat and may even cause fire due to the highly exothermic nature of such reactions. It is corrosive to metal and is very irritating – it may cause severe burns to the eye, skin,mucous membrane,andrespiratory tracteven at concentrations as low as 1 mg/m3.[11]
See also
[edit]References
[edit]- ^abGreenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.ISBN978-0-08-037941-8.
- ^Cruickshank, D. W. J. (1964)."Refinements of Structures Containing Bonds between Si, P, S or Cl and O or N: V. P4O10".Acta Crystallogr.17(6): 677–9.doi:10.1107/S0365110X64001669.
- ^D. E. C. Corbridge "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam.ISBN0-444-89307-5.
- ^ab.Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements".Inorganic Chemistry, 3rd Edition.Pearson. p. 473.ISBN978-0-13-175553-6.
- ^D. Stachel, I. Svoboda and H. Fuess (June 1995). "Phosphorus Pentoxide at 233 K".Acta Crystallogr. C.51(6): 1049–1050.doi:10.1107/S0108270194012126.
- ^Threlfall, Richard E., (1951).The story of 100 years of Phosphorus Making: 1851 - 1951.Oldbury: Albright & Wilson Ltd
- ^Meier, M. S. "Phosphorus(V) Oxide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York.doi:10.1002/047084289X.
- ^Joseph C. Salamone, ed. (1996).Polymeric materials encyclopedia: C, Volume 2.CRC Press. p. 1417.ISBN0-8493-2470-X.
- ^Tidwell, T. T. "Dimethyl Sulfoxide–Phosphorus Pentoxide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York.doi:10.1002/047084289X.
- ^Luer, B.; Jansen, M. "Crystal Structure Refinement of Tetraphosphorus Nonaoxide, P4O9"Zeitschrift für Kristallographie 1991, volume 197, pages 247-8.
- ^Phosphorus pentoxide MSDS