Cyclooxygenase-2
Cyclooxygenase-2(COX-2), also known asProstaglandin-endoperoxide synthase 2(HUGOPTGS2), is anenzymethat in humans is encoded by thePTGS2gene.[5]In humans it is one of threecyclooxygenases.It is involved in the conversion ofarachidonic acidtoprostaglandin H2,an important precursor ofprostacyclin,which is expressed ininflammation.
Function
[edit]PTGS2 (COX-2), convertsarachidonic acid (AA)to prostaglandin endoperoxide H2. PTGSs are targets forNSAIDsand PTGS2 (COX-2) specific inhibitors called coxibs. PTGS-2 is a sequence homodimer. Eachmonomerof the enzyme has aperoxidaseand a PTGS (COX)active site.The PTGS (COX) enzymes catalyze the conversion ofarachidonic acidtoprostaglandinsin two steps. First, hydrogen is abstracted from carbon 13 of arachidonic acid, and then two molecules of oxygen are added by the PTGS2 (COX-2), giving PGG2. Second,PGG2is reduced toPGH2in the peroxidase active site. The synthesized PGH2 is converted to prostaglandins (PGD2,PGE2,PGF2α),prostacyclin(PGI2), orthromboxane A2by tissue-specific isomerases (Figure 2).[6]
While metabolizing arachidonic acid primarily to PGG2, COX-2 also converts this fatty acid to small amounts of a racemic mixture of15-hydroxyicosatetraenoic acids(i.e., 15-HETEs) composed of ~22% 15(R)-HETE and ~78% 15(S)-HETEstereoisomersas well as a small amount of 11(R)-HETE.[7]The two 15-HETE stereoisomers have intrinsic biological activities but, perhaps more importantly, can be further metabolized to a major class of agents, thelipoxins.Furthermore,aspirin-treated COX-2 metabolizes arachidonic acid almost exclusively to 15(R)-HETE which product can be further metabolized to epi-lipoxins.[8]The lipoxins and epi-lipoxins are potent anti-inflammatory agents and may contribute to the overall activities of the two COX's as well as to aspirin.[citation needed]
COX-2 is naturally inhibited bycalcitriol(the active form of Vitamin D).[9][10]
Mechanism
[edit]
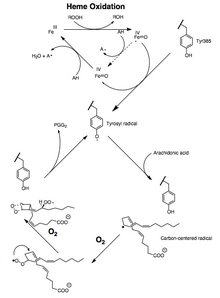
Both the peroxidase and PTGS activities are inactivated during catalysis by mechanism-based, first-order processes, which means that PGHS-2 peroxidase or PTGS activities fall to zero within 1–2 minutes, even in the presence of sufficient substrates.[12][13][14]
The conversion of arachidonic acid to PGG2 can be shown as a series ofradical reactionsanalogous to polyunsaturatedfatty acidautoxidation.[15]The 13-pro(S) -hydrogen is abstracted and dioxygen traps thepentadienylradical at carbon 11. The 11-peroxyl radical cyclizes at carbon 9 and the carbon-centered radical generated at C-8 cyclizes at carbon 12, generating theendoperoxide.Theallylicradical generated is trapped by dioxygen at carbon 15 to form the 15-(S) -peroxyl radical; this radical is then reduced toPGG2.This is supported by the following evidence: 1) a significantkinetic isotope effectis observed for the abstraction of the 13-pro (S)-hydrogen; 2) carbon-centered radicals are trapped duringcatalysis;[16]3) small amounts ofoxidationproducts are formed due to the oxygen trapping of an allylic radical intermediate at positions 13 and 15.[17][18]
Another mechanism in which the 13-pro (S)-hydrogen isdeprotonatedand thecarbanionisoxidizedto aradicalis theoretically possible. However, oxygenation of 10,10-difluoroarachidonic acid to 11-(S)-hydroxyeicosa-5,8,12,14-tetraenoic acid is not consistent with the generation of a carbanion intermediate because it would eliminate fluoride to form a conjugated diene.[19]The absence of endoperoxide-containing products derived from 10,10-difluoroarachidonic acid has been thought to indicate the importance of a C-10 carbocation inPGG2synthesis.[20]However, the cationic mechanism requires that endoperoxide formation comes before the removal of the 13-pro (S)-hydrogen. This is not consistent with the results of the isotope experiments ofarachidonic acidoxygenation.[21]
Structure
[edit]
PTGS2 (COX-2) exists as a homodimer, each monomer with a molecular mass of about 70 kDa. The tertiary and quaternary structures of PTGS1 (COX-1) and PTGS2 (COX-2) enzymes are almost identical. Each subunit has three different structural domains: a shortN-terminalepidermal growth factor (EGF) domain; anα-helicalmembrane-binding moiety; and aC-terminalcatalytic domain. PTGS (COX, which can be confused with "cytochrome oxidase") enzymes aremonotopicmembrane proteins; the membrane-binding domain consists of a series ofamphipathicα helices with severalhydrophobicamino acidsexposed to a membrane monolayer. PTGS1 (COX-1) and PTGS2 (COX-2) are bifunctional enzymes that carry out two consecutive chemical reactions in spatially distinct but mechanisticallycoupledactive sites. Both thecyclooxygenaseand theperoxidaseactive sites are located in the catalytic domain, which accounts for approximately 80% of the protein. The catalytic domain ishomologousto mammalian peroxidases such asmyeloperoxidase.[23][24]
It has been found that human PTGS2 (COX-2) functions as a conformational heterodimer having a catalytic monomer (E-cat) and an allosteric monomer (E-allo).Hemebinds only to theperoxidasesite of E-cat while substrates, as well as certaininhibitors(e.g.celecoxib), bind the COX site of E-cat. E-cat is regulated by E-allo in a way dependent on what ligand is bound to E-allo.Substrateand non-substratefatty acid(FAs) and some PTGS (COX) inhibitors (e.g.naproxen) preferentially bind to the PTGS (COX) site of E-allo.Arachidonic acidcan bind to E-cat and E-allo, but the affinity of AA for E-allo is 25 times that for Ecat. Palmitic acid, an efficacious stimulator ofhuPGHS-2,binds only E-allo in palmitic acid/murine PGHS-2 co-crystals. Non-substrate FAs can potentiate orattenuatePTGS (COX) inhibitors depending on thefatty acidand whether the inhibitor binds E-cat or E-allo. Studies suggest that the concentration and composition of the free fatty acid pool in the environment in which PGHS-2 functions in cells, also referred to as the FA tone, is a key factor regulating the activity of PGHS-2 and its response to PTGS (COX) inhibitors.[22]
Clinical significance
[edit]
PTGS2 (COX-2) is unexpressed under normal conditions in most cells, but elevated levels are found duringinflammation.PTGS1 (COX-1) is constitutively expressed in many tissues and is the predominant form in gastric mucosa and in the kidneys. Inhibition of PTGS1 (COX-1) reduces thebasal productionof cytoprotectivePGE2andPGI2in thestomach,which may contribute togastric ulceration.Since PTGS2 (COX-2) is generally expressed only in cells whereprostaglandinsare upregulated (e.g., during inflammation), drug-candidates that selectively inhibit PTGS2 (COX-2) were suspected to show fewerside-effects[24]but proved to substantially increase risk for cardiovascular events such as heart attack and stroke. Two different mechanisms may explain contradictory effects. Low-dose aspirin protects against heart attacks and strokes by blocking PTGS1 (COX-1) from forming a prostaglandin called thromboxane A2. It sticks platelets together and promotes clotting; inhibiting this helps prevent heart disease. On the other hand, PTGS2 (COX-2) is a more important source of prostaglandins, particularly prostacyclin which is found in blood vessel lining. Prostacyclin relaxes or unsticks platelets, soselective COX-2 inhibitors(coxibs) increase risk of cardiovascular events due to clotting.[26]
Non-steroidal anti-inflammatory drugs(NSAIDs) inhibitprostaglandinproduction by PTGS1 (COX-1) and PTGS2 (COX-2).NSAIDsselective for inhibition of PTGS2 (COX-2) are less likely than traditional drugs to causegastrointestinaladverse effects, but could causecardiovascularevents, such asheart failure,myocardial infarction,andstroke.Studies with humanpharmacologyandgenetics,genetically manipulatedrodents,and other animal models and randomized trials indicate that this is due to suppression of PTGS2 (COX-2)-dependent cardioprotectiveprostaglandins,prostacyclinin particular.[27]
The expression of PTGS2 (COX-2) is upregulated in many cancers. The overexpression of PTGS2 (COX-2) along with increased angiogenesis and SLC2A1 (GLUT-1) expression is significantly associated with gallbladder carcinomas.[28]Furthermore, the product of PTGS2 (COX-2),PGH2is converted byprostaglandin E2 synthaseintoPGE2,which in turn can stimulate cancer progression. Consequently, inhibiting PTGS2 (COX-2) may have benefit in the prevention and treatment of these types of cancer.[29][30]
COX-2 expression was found in human idiopathic epiretinal membranes.[31]Cyclooxygenases blocking bylornoxicamin acute stage of inflammation reduced the frequency of membrane formation by 43% in thedispasemodel ofPVRand by 31% in theconcanavalinone.Lornoxicamnot only normalized the expression of cyclooxygenases in both models of PVR, but also neutralized the changes of theretinaand thechoroidthickness caused by the injection of pro-inflammatory agents. These facts underline the importance of cyclooxygenases and prostaglandins in the development of PVR.[32]
PTGS2 gene upregulation has also been linked with multiple stages of human reproduction. Presence of gene is found in thechorionic plate,in theamnion epithelium,syncytiotrophoblasts,villous fibroblasts, chorionictrophoblasts,amniotic trophoblasts,as well as thebasal plate of the placenta,in thedecidual cellsandextravillous cytotrophoblasts.During the process ofchorioamnionitis/deciduitis, the upregulation of PTGS2 in theamnionand choriodecidua is one of three limited effects of inflammation in theuterus.Increased expression of the PTGS2 gene in thefetal membranesis connected to the presence of inflammation, causing uterine prostaglandin gene expression and immunolocalization ofprostaglandinpathway proteins in chorionic trophoblast cells and adjacent decidua, or choriodecidua. PTGS2 is linked with the inflammatory system and has been observed in inflammatoryleukocytes.It has been noted that there is a positive correlation with PTGS2 expression in the amnion during spontaneous labour and was discovered to have increased expression with gestational age following the presence of labour with no change observed in amnion and choriodecidua during either preterm or term labour. Additionally,oxytocinstimulates the expression of PTGS2 inmyometrial cells.[33]
The mutant allele PTGS2 5939C carriers among the Han Chinese population have been shown to have a higher risk ofgastric cancer.In addition, a connection was found betweenHelicobacter pyloriinfection and the presence of the 5939C allele.[34]
Interactions
[edit]PTGS2 has been shown tointeractwithcaveolin 1.[35]
History
[edit]PTGS2 (COX-2) was discovered in 1991 by theDaniel Simmonslaboratory[36][better source needed]at Brigham Young University.
See also
[edit]- Arachidonic acid
- Cyclooxygenase
- Cyclooxygenase 1
- NSAID
- Discovery and development of COX-2 selective inhibitors
- COX-2 selective inhibitor
References
[edit]- ^abcGRCh38: Ensembl release 89: ENSG00000073756–Ensembl,May 2017
- ^abcGRCm38: Ensembl release 89: ENSMUSG00000032487–Ensembl,May 2017
- ^"Human PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^"Mouse PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^Hla T, Neilson K (August 1992)."Human cyclooxygenase-2 cDNA".Proc. Natl. Acad. Sci. U.S.A.89(16): 7384–8.Bibcode:1992PNAS...89.7384H.doi:10.1073/pnas.89.16.7384.PMC49714.PMID1380156.
- ^O'Banion MK (1999). "Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology".Crit Rev Neurobiol.13(1): 45–82.doi:10.1615/critrevneurobiol.v13.i1.30.PMID10223523.
- ^Mulugeta S, Suzuki T, Hernandez NT, Griesser M, Boeglin WE, Schneider C (2010)."Identification and absolute configuration of dihydroxy-arachidonic acids formed by oxygenation of 5S-HETE by native and aspirin-acetylated COX-2".J. Lipid Res.51(3): 575–85.doi:10.1194/jlr.M001719.PMC2817587.PMID19752399.
- ^Serhan CN (2005). "Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution".Prostaglandins Leukot. Essent. Fatty Acids.73(3–4): 141–62.doi:10.1016/j.plefa.2005.05.002.PMID16005201.
- ^Wang Q, He Y, Shen Y, Zhang Q, Chen D, Zuo C, Qin J, Wang H, Wang J, Yu Y (2014)."Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4".J Biol Chem.289(17): 11681–11694.doi:10.1074/jbc.M113.517581.PMC4002078.PMID24619416.
- ^Kassi E, Adamopoulos C, Basdra EK, Papavassiliou AG (2013)."Role of Vitamin D in Atherosclerosis".Circulation.128(23): 2517–2531.doi:10.1161/CIRCULATIONAHA.113.002654.PMID24297817.
- ^PDB:3OLT
- ^Smith WL, Garavito RM, DeWitt DL (December 1996)."Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2".J. Biol. Chem.271(52): 33157–60.doi:10.1074/jbc.271.52.33157.PMID8969167.
- ^Wu G, Wei C, Kulmacz RJ, Osawa Y, Tsai AL (April 1999)."A mechanistic study of self-inactivation of the peroxidase activity in prostaglandin H synthase-1".J. Biol. Chem.274(14): 9231–7.doi:10.1074/jbc.274.14.9231.PMID10092596.
- ^Callan OH, So OY, Swinney DC (February 1996)."The kinetic factors that determine the affinity and selectivity for slow binding inhibition of human prostaglandin H synthase 1 and 2 by indomethacin and flurbiprofen".J. Biol. Chem.271(7): 3548–54.doi:10.1074/jbc.271.7.3548.PMID8631960.
- ^Porter NA (1986). "Mechanisms for the autoxidation of polyunsaturated lipids".Accounts of Chemical Research.19(9): 262–8.doi:10.1021/ar00129a001.
- ^Mason RP, Kalyanaraman B, Tainer BE, Eling TE (June 1980)."A carbon-centered free radical intermediate in the prostaglandin synthetase oxidation of arachidonic acid. Spin trapping and oxygen uptake studies".J. Biol. Chem.255(11): 5019–22.doi:10.1016/S0021-9258(19)70741-8.PMID6246094.
- ^Hecker M, Ullrich V, Fischer C, Meese CO (November 1987)."Identification of novel arachidonic acid metabolites formed by prostaglandin H synthase".Eur. J. Biochem.169(1): 113–23.doi:10.1111/j.1432-1033.1987.tb13587.x.PMID3119336.
- ^Xiao G, Tsai AL, Palmer G, Boyar WC, Marshall PJ, Kulmacz RJ (February 1997). "Analysis of hydroperoxide-induced tyrosyl radicals and lipoxygenase activity in aspirin-treated human prostaglandin H synthase-2".Biochemistry.36(7): 1836–45.doi:10.1021/bi962476u.PMID9048568.
- ^Kwok PY, Muellner FW, Fried J (June 1987). "Enzymatic conversions of 10,10-difluoroarachidonic acid with PGH synthase and soybean lipoxygenase".Journal of the American Chemical Society.109(12): 3692–3698.doi:10.1021/ja00246a028.
- ^Dean AM, Dean FM (May 1999)."Carbocations in the synthesis of prostaglandins by the cyclooxygenase of PGH synthase? A radical departure!".Protein Sci.8(5): 1087–98.doi:10.1110/ps.8.5.1087.PMC2144324.PMID10338019.
- ^Hamberg M, Samuelsson B (November 1967)."On the mechanism of the biosynthesis of prostaglandins E-1 and F-1- Alpha".J. Biol. Chem.242(22): 5336–43.doi:10.1016/S0021-9258(18)99433-0.PMID6070851.
- ^abDong L, Vecchio AJ, Sharma NP, Jurban BJ, Malkowski MG, Smith WL (May 2011)."Human cyclooxygenase-2 is a sequence homodimer that functions as a conformational heterodimer".J. Biol. Chem.286(21): 19035–46.doi:10.1074/jbc.M111.231969.PMC3099718.PMID21467029.
- ^Picot D, Loll PJ, Garavito RM (January 1994). "The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1".Nature.367(6460): 243–9.Bibcode:1994Natur.367..243P.doi:10.1038/367243a0.PMID8121489.S2CID4340064.
- ^abKurumbail RG, Kiefer JR, Marnett LJ (December 2001). "Cyclooxygenase enzymes: catalysis and inhibition".Curr. Opin. Struct. Biol.11(6): 752–60.doi:10.1016/S0959-440X(01)00277-9.PMID11751058.
- ^PDB:3PGH
- ^Ruan CH, So SP, Ruan KH (2011)."Inducible COX-2 dominates over COX-1 in prostacyclin biosynthesis: Mechanisms of COX-2 inhibitor risk to heart disease".Life Sciences.88(1–2): 24–30.doi:10.1016/j.lfs.2010.10.017.PMC3046773.PMID21035466.
- ^Wang D, Patel VV, Ricciotti E, Zhou R, Levin MD, Gao E, Yu Z, Ferrari VA, Lu MM, Xu J, Zhang H, Hui Y, Cheng Y, Petrenko N, Yu Y, FitzGerald GA (May 2009)."Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function".Proc. Natl. Acad. Sci. U.S.A.106(18): 7548–52.Bibcode:2009PNAS..106.7548W.doi:10.1073/pnas.0805806106.PMC2670242.PMID19376970.
- ^Legan M (August 2010)."Cyclooxygenase-2, p53 and glucose transporter-1 as predictors of malignancy in the development of gallbladder carcinomas".Bosn J Basic Med Sci.10(3): 192–6.doi:10.17305/bjbms.2010.2684.PMC5504494.PMID20846124.
- ^EntrezGene5743
- ^Menter DG, Schilsky RL, DuBois RN (March 2010)."Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward".Clin. Cancer Res.16(5): 1384–90.doi:10.1158/1078-0432.CCR-09-0788.PMC4307592.PMID20179228.
- ^KASE S, SAITO W, OHNO S, ISHIDA S (2010). "Cyclo-Oxygenase-2 Expression in Human Idiopathic Epiretinal Membrane".Retina.30(5): 719–723.doi:10.1097/iae.0b013e3181c59698.PMID19996819.S2CID205650971.
- ^Tikhonovich MV, Erdiakov AK, Gavrilova SA (2017-06-21). "Nonsteroid anti-inflammatory therapy suppresses the development of proliferative vitreoretinopathy more effectively than a steroid one".International Ophthalmology.38(4): 1365–1378.doi:10.1007/s10792-017-0594-3.ISSN0165-5701.PMID28639085.S2CID4017540.
- ^Phillips, Robert J et al. "Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation." BMC pregnancy and childbirth vol. 14 241. 22 Jul. 2014, doi:10.1186/1471-2393-14-241
- ^Li Y, He W, Liu T, Zhang Q (December 2010). "A new cyclo-oxygenase-2 gene variant in the Han Chinese population is associated with an increased risk of gastric carcinoma".Mol Diagn Ther.14(6): 351–5.doi:10.1007/bf03256392.PMID21275453.S2CID1229751.
- ^Liou JY, Deng WG, Gilroy DW, Shyue SK, Wu KK (September 2001)."Colocalization and interaction of cyclooxygenase-2 with caveolin-1 in human fibroblasts".J. Biol. Chem.276(37): 34975–82.doi:10.1074/jbc.M105946200.PMID11432874.
- ^Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL (April 1991)."Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing".Proc. Natl. Acad. Sci. U.S.A.88(7): 2692–6.Bibcode:1991PNAS...88.2692X.doi:10.1073/pnas.88.7.2692.PMC51304.PMID1849272.
Further reading
[edit]- Richards JA, Petrel TA, Brueggemeier RW (February 2002). "Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells".J. Steroid Biochem. Mol. Biol.80(2): 203–12.doi:10.1016/S0960-0760(01)00187-X.PMID11897504.S2CID12728545.
- Wu T, Wu H, Wang J, Wang J (2011)."Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain".J Neuroinflammation.8:22.doi:10.1186/1742-2094-8-22.PMC3062590.PMID21385433.
- Koki AT, Khan NK, Woerner BM, Seibert K, Harmon JL, Dannenberg AJ, Soslow RA, Masferrer JL (January 2002). "Characterization of cyclooxygenase-2 (COX-2) during tumorigenesis in human epithelial cancers: evidence for potential clinical utility of COX-2 inhibitors in epithelial cancers".Prostaglandins Leukot. Essent. Fatty Acids.66(1): 13–8.doi:10.1054/plef.2001.0335.PMID12051953.
- Saukkonen K, Rintahaka J, Sivula A, Buskens CJ, Van Rees BP, Rio MC, Haglund C, Van Lanschot JJ, Offerhaus GJ, Ristimaki A (October 2003). "Cyclooxygenase-2 and gastric carcinogenesis".APMIS.111(10): 915–25.doi:10.1034/j.1600-0463.2003.1111001.x.PMID14616542.S2CID23257867.
- Sinicrope FA, Gill S (2004). "Role of cyclooxygenase-2 in colorectal cancer".Cancer Metastasis Rev.23(1–2): 63–75.doi:10.1023/A:1025863029529.PMID15000150.S2CID21521040.
- Jain S, Khuri FR, Shin DM (2004). "Prevention of head and neck cancer: current status and future prospects".Curr Probl Cancer.28(5): 265–86.doi:10.1016/j.currproblcancer.2004.05.003.PMID15375804.
- Saba N, Jain S, Khuri F (2004). "Chemoprevention in lung cancer".Curr Probl Cancer.28(5): 287–306.doi:10.1016/j.currproblcancer.2004.05.005.PMID15375805.
- Cardillo I, Spugnini EP, Verdina A, Galati R, Citro G, Baldi A (October 2005). "Cox and mesothelioma: an overview".Histol. Histopathol.20(4): 1267–74.PMID16136507.
- Brueggemeier RW, Díaz-Cruz ES (March 2006). "Relationship between aromatase and cyclooxygenases in breast cancer: potential for new therapeutic approaches".Minerva Endocrinol.31(1): 13–26.PMID16498361.
- Fujimura T, Ohta T, Oyama K, Miyashita T, Miwa K (March 2006)."Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: a review and report of personal experience".World J. Gastroenterol.12(9): 1336–45.doi:10.3748/wjg.v12.i9.1336.PMC4124307.PMID16552798.
- Bingham S, Beswick PJ, Blum DE, Gray NM, Chessell IP (October 2006). "The role of the cylooxygenase pathway in nociception and pain".Semin. Cell Dev. Biol.17(5): 544–54.doi:10.1016/j.semcdb.2006.09.001.PMID17071117.
- Minghetti L, Pocchiari M (2007).Cyclooxygenase-2, prostaglandin E2, and microglial activation in prion diseases.International Review of Neurobiology. Vol. 82. pp. 265–75.doi:10.1016/S0074-7742(07)82014-9.ISBN978-0-12-373989-6.PMID17678966.
External links
[edit]- NextbioArchived2019-10-18 at theWayback Machine
- NSAIDs and Cardiovascular Risk Explained, According to Studies from the Perelman School of Medicine
- Wolfe MM (December 2004). "Rofecoxib, Merck, and the FDA".N. Engl. J. Med.351(27): 2875–8, author reply 2875–8.doi:10.1056/NEJM200412303512719.PMID15625749.






