Plutonium in the environment
| Part of aserieson |
| Pollution |
|---|
 |
Since the mid-20th century,plutonium in the environmenthas been primarily produced by human activity. The first plants to produceplutoniumfor use incold waratomic bombswere theHanford nuclear site,in Washington, andMayaknuclear plant, inChelyabinsk Oblast,Russia. Over a period of four decades,[1]"both released more than 200 million curies of radioactive isotopes into the surrounding environment – twice the amount expelled in theChernobyl disasterin each instance. "[2]
The majority ofplutoniumisotopesare short-lived on ageological timescale,[3]though it has been argued that traces of the long-lived244Puisotope still exist in nature.[4]This isotope has been found inlunar soil,[5]meteorites,[6]and in theOklonatural reactor.[7]However, one study on plutonium inmarinesedimentsindicates that the atomic bomb fallout accounts for 66% of the239Puand 59%240Puin theEnglish Channel.In contrast,nuclear reprocessingcontributes the majority of the238Puand241Puin the Earth's oceans, whereas nuclear weapons testing is responsible for only 6.5% and 16.5% of these isotopes, respectively.[8]
Sources of plutonium[edit]
Plutonium production[edit]

Richland, Washingtonwas the first city established to support plutonium production at the nearbyHanford nuclear site,to power the American nuclear weapons arsenals.Ozersk, Russiasupported plutonium production to power the Soviet nuclear arsenals at theMayaknuclear plant. These were the first two cities in the world to produce plutonium for use incold waratomic bombs.[2]
In the 2013 book,Plutopia: Nuclear Families, Atomic Cities, and the Great Soviet and American Plutonium Disasters,Kate Brownexplores the health of affected citizens in both the United States and Russia, and the "slow-motion disasters" that still threaten the environments where the plants are located.[1]According to Brown, the plants at Hanford and Mayak released over 200 millioncuriesof radioactive isotopes into the surrounding environment over four decades, which is twice the amount expelled in theChernobyl disasterin each instance.[2]
Most of theradioactive contaminationover the years from Hanford and Mayak were part of normal operations. Unforeseen accidents did occur, but plant management kept this secret, and the pollution continued unabated. Even today, as pollution threats to health and the environment persist, the government conceals information about the associated risks from the public.[2]
Bomb detonations[edit]
About 3.5 tons of plutonium have been released into the environment by atomic bomb tests. While this might sound significant, it has only resulted in a very small dose to the majority of the humans on earth. Overall the health effects offission productsare far greater than the effects of theactinidesreleased by a nuclear bomb detonation. The plutonium from the fuel of the bomb is converted into a high-firedoxidethat is carried high into the air. It slowly falls to earth as globalfalloutand is notsoluble,and as a result it is difficult for this plutonium to be incorporated into an organism if ingested. Much of this plutonium is absorbed into sediments of lakes, rivers and oceans. However, about 66% of the plutonium from a bomb explosion is formed by the neutron capture of uranium-238; this plutonium is not converted by the bomb into a high fired oxide as it is formed more slowly. This formed plutonium is more soluble and more harmful as fallout.[9]
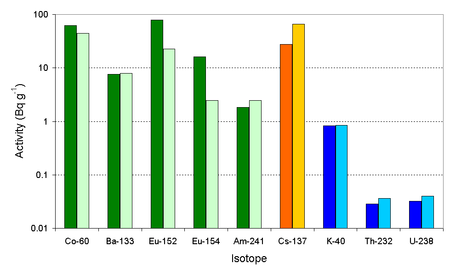
Some plutonium can be deposited close to the point of detonation. The glassytrinititeformed by theTrinity bombhas been examined to determine what actinides and otherradioisotopesit contained. A 2006 paper[10]reports the levels of long lived radioisotopes in the trinitite.152Eu and154Eu was mainly formed by the neutron activation of theeuropiumin the soil, and the level of radioactivity for these isotopes is highest where the neutron dose to thesoilwas larger. Some of the60Co was generated by activation of thecobaltin the soil, but some was also generated by the activation of the cobalt in thesteel(100 foot) tower on which the bomb stood. This60Co from the tower would have been scattered over the site reducing the difference in the soil levels.133Ba and241Amwere created by the neutron activation of barium and plutonium inside the bomb. Thebariumwas present in the form of thenitratein the chemical explosives used while the plutonium was thefissilefuel used.
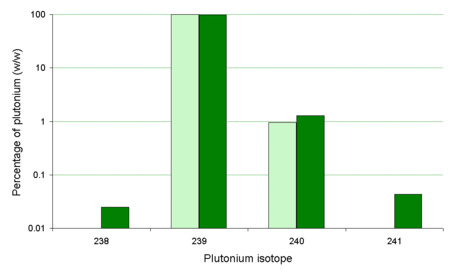
As the239Pu/240Pu ratio only changed slightly during the Trinity detonation, it has been commented[11]that this isotope ratio for the majority of atomic bombs (in Japan the239Pu/240Pu ratio in soil is normally in the range 0.17 to 0.19[12]) is very different than from the bomb dropped uponNagasaki.
Bomb safety trials[edit]

Plutonium has also been released into the environment insafety trials.In these experiments,nuclear bombshave been subjected to simulated accidents or detonated with an abnormal initiation of their chemical explosives. An abnormal implosion will result in a compression of theplutonium pit,which is less uniform and smaller than the designed compression in the device. In these experiments where no or very littlenuclear fissionoccurs, plutonium metal has been scattered around the test sites. While some of these tests have been done underground, other such tests were conducted in open air. A paper on theradioisotopesleft on an island by theFrenchnuclear bombs tests of the 20th century has been printed by theInternational Atomic Energy Agencyand a section of this report deals with plutonium contamination resulting from such tests.[13]
Other related trials were conducted atMaralinga, South Australiawhere both normal bomb detonations and "safety trials" have been conducted. While the activity from the fission products has decayed away almost totally (as of 2006) the plutonium remains active.[14][15]
Space[edit]
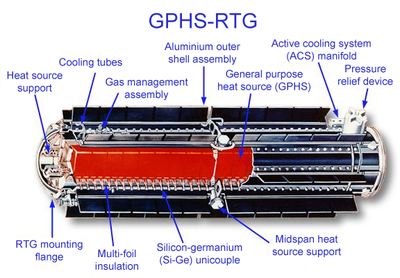
Plutonium can also be introduced into the environment via the reentry of artificial satellites containingatomic batteries.There have been several such incidents, the most prominent being theApollo 13mission. TheApollo Lunar Surface Experiments Packagecarried on theLunar Modulere-entered the atmosphere over the South Pacific. Many atomic batteries have been of theradioisotope thermoelectric generator(RTG) type. The Plutonium-238 used in RTGs has ahalf-lifeof 88 years, as opposed to the plutonium-239 used innuclear weaponsandreactors,which has ahalf-lifeof 24,100 years.[full citation needed]In April 1964 aSNAP-9Afailed to achieve orbit and disintegrated, dispersing roughly 1 kilogram (2.2 lb) ofplutonium-238over all continents. Most plutonium fell in the southern hemisphere. An estimated 6300GBqor 2100 man-Sv of radiation was released[16][17][18][19]and led to NASA's development of solar photovoltaic energy technology.[20][better source needed]

Chain reactions do not occur inside RTGs, so anuclear meltdownis impossible. In fact, some RTGs are designed so that fission does not occur at all; rather, forms ofradioactive decaywhich cannot trigger other radioactive decays are used instead. As a result, the fuel in an RTG is consumed much more slowly and much less power is produced. RTGs are still a potential source ofradioactive contamination:if the container holding the fuel leaks, the radioactive material will contaminate the environment. The main concern is that if an accident were to occur during launch or a subsequent passage of a spacecraft close to Earth, harmful material could be released into the atmosphere. However, this event is extremely unlikely with current RTG cask designs.[full citation needed]
In order to minimise the risk of the radioactive material being released, the fuel is typically stored in individual modular units with their own heat shielding. They are surrounded by a layer ofiridiummetal and encased in high-strengthgraphiteblocks. These two materials are corrosion and heat-resistant. Surrounding the graphite blocks is an aeroshell, designed to protect the entire assembly against the heat of reentering the Earth's atmosphere. The plutonium fuel is also stored in a ceramic form that is heat-resistant, minimising the risk of vaporization and aerosolization. The ceramic is also highlyinsoluble.[full citation needed]
The US Department of Energy has conducted seawater tests and determined that the graphite casing, which was designed to withstand reentry, is stable and no release of plutonium should occur. Subsequent investigations have found no increase in the natural background radiation in the area. The Apollo 13 accident represents an extreme scenario due to the high re-entry velocities of the craft returning from cislunar space. This accident has served to validate the design of later-generation RTGs as highly safe.
Nuclear fuel cycle[edit]
Plutonium has been released into the environment in aqueous solution fromnuclear reprocessinganduranium enrichmentplants. The chemistry of this plutonium is different from that of the metal oxides formed fromnuclear bombdetonations.
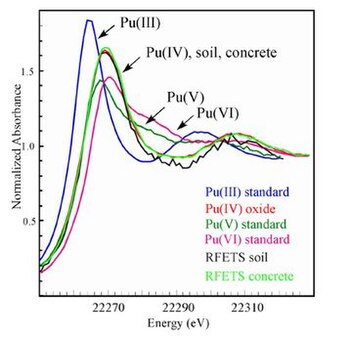
One example of a site where plutonium entered the soil isRocky Flatswhere in the recent pastXANES(X-rayspectroscopy) has been used to determine the chemical nature of the plutonium in thesoil.[21]The XANES was used to determine theoxidation stateof the plutonium, whileEXAFSwas used to investigate the structure of the plutonium compound present in the soil andconcrete.[22]
Chernobyl[edit]
Because plutonium oxide is very involatile, most of the plutonium in the reactor was not released during the fire. However that which was released can be measured. V.I. Yoschenko et al. reported that grass and forest fires can make thecaesium,strontiumandplutoniumbecome mobile in the air again.[23]
Fukushima[edit]
The ongoing crisis at this site includes Spent Fuel Pools on the upper floors, exposed to the elements with complex MOX and plutonium products. The Japanese Government Taskforce has asked for submissions to the International Research Institute for Nuclear Decommissioning[24]in regards to the ongoing Contaminated Water Issues.[25]
Nuclear crime[edit]
There have been 18 incidences[spelling?]of theft or loss ofhighly enriched uranium(HEU) andplutoniumconfirmed by the IAEA.[26]
One case exists of aGermanman who attempted to poison his ex-wife with plutonium stolen from WAK (WiederaufbereitungsanlageKarlsruhe), a small scale reprocessing plant where he worked. He did not steal a large amount of plutonium, just rags used for wiping surfaces and a small amount of liquid waste. The man was sent toprisonfor his crime.[27]At least two other people were contaminated by the plutonium.[citation needed]Two flats inRhineland-Palatinatewere also contaminated.[28]These were later cleaned at a cost of two millioneuro.
Environmental chemistry[edit]
Overview[edit]
Plutonium, like other actinides, readily forms a dioxideplutonylcore (PuO2). In the environment, this plutonyl core readily complexes with carbonate as well as other oxygenmoieties(OH−,NO2−,NO3−,and SO42−) to form charged complexes which can be readily mobile with low affinities to soil.[citation needed]
- PuO2(CO3)12−
- PuO2(CO3)24−
- PuO2(CO3)36−
PuO2formed from neutralizing highly acidic nitric acid solutions tends to form polymeric PuO2which is resistant to complexation. Plutonium also readily shifts valences between the +3, +4, +5 and +6 states. It is common for some fraction of plutonium in solution to exist in all of these states in equilibrium.[citation needed]
Binding to soil[edit]
Plutonium is known to bind to soil particles very strongly (see above for an X-ray spectroscopic study of plutonium in soil andconcrete). Whilecaesiumhas very different chemistry to the actinides, it is well known that both caesium and many of the actinides bind strongly to themineralsin soil. Hence it has been possible to use134Cs labeled soil to study the migration of Pu and Cs in soils. It has been shown thatcolloidaltransport processes control the migration of Cs (and will control the migration of Pu) in the soil at theWaste Isolation Pilot Plantaccording to R.D. Whicker and S.A. Ibrahim.[29]J.D. Chaplin et al. recently reported advances in theDiffusive gradients in thin filmstechnique, which have provided a method to measure labile bioavailablePlutoniumin soils, as well as in freshwater and seawater.[30]
Microbiological chemistry[edit]
Mary Neu (atLos Alamosin the USA) has done some work which suggests thatbacteriacan accumulate plutonium because theirontransport systems used by the bacteria also function as plutonium transport systems.[31][32][33]
Biology[edit]
Plutonium ingested by or injected into humans is transported in thetransferrinbasediron(III) transport system and then is stored in theliverin the iron store (ferritin), after an exposure to plutonium it is important to rapidly inject the subject with achelatingagent such ascalciumcomplex[34]ofDTPA.[35][36]This antidote is useful for a single exposure such as that which would occur if aglove boxworker were to cut his or her hand with a plutonium-contaminated object. The calcium complex has faster metal binding kinetics than thezinccomplex but if the calcium complex is used for a long time it tends to remove important minerals from the person. The zinc complex is less able to cause these effects.
Plutonium that is inhaled by humans lodges in the lungs and is slowly translocated to thelymph nodes.Inhaled plutonium has been shown to lead to lung cancer in experimental animals.[37]
See also[edit]
References[edit]
- ^abBrown, Kate(2013).Plutopia: nuclear families, atomic cities, and the great Soviet and American plutonium disasters.Oxford:Oxford University Press.ISBN978-0-19-985576-6.
- ^abcdRobert Lindley (2013)."Kate Brown: Nuclear" Plutopias "the Largest Welfare Program in American History".History News Network.
- ^"Plutonium"(PDF).Human Health Fact Sheet.Argonne National Laboratory, EVS. August 2005. Archived fromthe original(PDF)on 2009-02-25.Retrieved2009-07-06.
- ^P.K. Kuroda,Accounts of Chemical Research,1979,12(2), 73-78[1]
- ^Kuroda, P. K.,and Myers, W. A. "Plutonium-244 Dating III Initial Ratios of Plutonium to Uranium in Lunar Samples".Journal of Radioanalytical and Nuclear Chemistry150, 71.
- ^Myers, W. A., andKuroda, P. K."Plutonium-244 Dating IV. Initial Ratios of Plutonium to Uranium in the Renazzo, Mokoia and Groznaya Meteorite".Journal of Radioanalytical and Nuclear Chemistry152, 99.
- ^Kuroda, P. K."Plutonium-244 in the Early Solar System and the Pre-Fermi Natural Reactor"(The Shibata Prize Awardee's Lecture).Geochemical Journal26, 1. (1992)
- ^O. F. X. Donard, F. Bruneau, M. Moldovan, H. Garraud, V. N. Epov, and D. Boust.Analytica Chimica Acta2007,587,170–179
- ^Radiochemistry and Nuclear Chemistry, G. Choppin, J-O. Liljenzin and J. Rydberg, 3rd Ed, Butterworth-Heinemann, 2002
- ^P.P. Parekh, T.M. Semkow, M.A. Torres, D.K. Haines, J.M. Cooper, P.M. Rosenberg and M.E. Kitto,Journal of Environmental Radioactivity,2006,85,103-120
- ^Y. Saito-Kokubu, F. Esaka, K. Yasuda, M. Magara, Y. Miyamoto, S. Sakurai, S. Usuda, H. Yamazaki, S. Yoshikawa and S. Nagaoka,Applied Radiation and Isotopes,2007,65(4), 465-468
- ^S. Yoshida, Y. Muramatsu, S. Yamazaki and T. Ban-nai,Journal of Environmental Radioactivity,2007, In Pressdoi:10.1016/j.jenvrad.2007.01.019
- ^The Radiological Situation at the Atolls of Mururoa and Fangataufa(PDF).International Atomic Energy Agency.1998.ISBN92-0-101198-9.Retrieved2009-07-06.
- ^"Resources (martac report)"(PDF).Archived fromthe original(PDF)on 2011-03-28.
- ^"Alan Parkinson - 2000 National Conference - MAPW Australia".Archived fromthe originalon 2008-02-01.
- ^Attila Vértes,Sándor Nagy, Zoltan Klencsár, Rezső G. Lovas"Handbook of nuclear chemistry", published 2003
- ^"Energy, Waste and the Environment: A Geochemical Perspective" author R. Gieré, Peter Stille. Page 145.
- ^Emergency Preparedness for Nuclear Powered Satellites.Stockholm: Organisation for Economic Co-operation and Development. 1990. p. 21.ISBN9264133526.
- ^Hardy, E. P.; Krey, P. W. & Volchok, H. L. (1972).Global inventory and distribution of Pu-238 from SNAP-9A(PDF).United States Atomic Energy Commission. p. 6.doi:10.2172/4689831.
- ^Grossman, Karl."Nukes In Space in Wake of Columbia Tragedy".Hieronymous & Company.Retrieved27 August2012.
- ^Clark, David L. (2002-05-29)."Cleanup at Rocky Flats".Los Alamos National Laboratory.Stanford Synchrotron Radiation Lightsource.Retrieved2009-07-06.
- ^"Plutonium Contamination Valence State Determination Using X-ray Absorption Fine Structure Permits Concrete Recycle"(PDF).Archived fromthe original(PDF)on 20 October 2011.
- ^(Journal of Environmental Radioactivity,2006,86,143-163.)
- ^"International Research Institute for Nuclear Decommissioning┃TOP Page".Archived fromthe originalon 2013-10-16.Retrieved2013-10-13.
- ^"Request for Information (RFI) for Contaminated Water Issues".
- ^Bunn, Matthew& Col-Gen. E.P. Maslin (2010)."All Stocks of Weapons-Usable Nuclear Materials Worldwide Must be Protected Against Global Terrorist Threats"(PDF).Belfer Center for Science and International Affairs, Harvard University.RetrievedJuly 26,2012.
- ^"Wise Nc; Germany: Plutonium Soup As A Murder Weapon?".WISE News Communique.2001-10-05.Retrieved2009-07-06.
- ^Hoefer, Hagen."Clean-up of a GBq-Pu contamination of two apartments, contaminated by the Pu theft at the WAK (Pilot Reprocessing Plant - Karlsruhe)"(PDF).
- ^Journal of Environmental Radioactivity,2006,88,171-188.
- ^Chaplin J, Warwick P, Cundy A, Bochud F, Froidevaux P (25 August 2021)."Novel DGT Configurations for the Assessment of Bioavailable Plutonium, Americium, and Uranium in Marine and Freshwater Environments".Analytical Chemistry.93(35): 11937–11945.doi:10.1021/acs.analchem.1c01342.PMID34432435.S2CID237307309.
- ^Neu, Mary P. (November 26, 2000)."Siderophore-Mediated Chemistry and Microbial Uptake of Plutonium"(PDF).Chemical Interactions of Actinides in the Environment.Los Alamos Science: 416–417.Retrieved2009-07-06.
- ^John SG, Ruggiero CE, Hersman LE, Tung CS, Neu MP (July 2001). "Siderophore mediated plutonium accumulation by Microbacterium flavescens (JG-9)".Environ. Sci. Technol.35(14): 2942–8.Bibcode:2001EnST...35.2942J.doi:10.1021/es010590g.PMID11478246.
- ^"Bacterial Biotransformations for the In situ Stabilization of Plutonium"(PDF).April 2005.Retrieved2009-07-06.
- ^"Pentetate calcium trisodium injection (Ca-DTPA)".Cerner Multum. Archived fromthe originalon September 28, 2007.Retrieved2009-07-06.
- ^ORISE: Radiation Emergency Assistance Center/Training Site
- ^"Pentetate zinc trisodium injection (Zn-DTPA)".Cerner Multum. Archived fromthe originalon September 28, 2007.Retrieved2009-07-06.
- ^"CDC Radiation Emergencies | Radioisotope Brief: Plutonium-239 (Pu-239)".cdc.gov.2022-04-21.Retrieved2022-06-17.
