Polythiophene



Polythiophenes(PTs) arepolymerizedthiophenes,asulfurheterocycle.The parent PT is an insoluble colored solid with the formula (C4H2S)n.[notes 1][2][3]The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s havealkylsubstituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
PTs becomeconductivewhen oxidized. Theelectrical conductivityresults from thedelocalization of electronsalong the polymer backbone. Conductivity however is not the only interesting property resulting from electron delocalization. The optical properties of these materials respond to environmental stimuli, with dramatic color shifts in response to changes insolvent,temperature,applied potential,and binding to other molecules. Changes in both color and conductivity are induced by the same mechanism, twisting of the polymer backbone and disrupting conjugation, making conjugated polymers attractive assensorsthat can provide a range of optical and electronic responses.[4][5][6]
The development of polythiophenes and related conductive organic polymers was recognized by the awarding of the 2000Nobel Prize in ChemistrytoAlan J. Heeger,Alan MacDiarmid,andHideki Shirakawa"for the discovery and development of conductive polymers".
Mechanism of conductivity and doping
[edit]PT is an ordinary organic polymer, being a red solid that is poorly soluble in most solvents.[7]Upon treatment with oxidizing agents (electron-acceptors) however, the material takes on a dark color and becomes electrically conductive. Oxidation is referred to as "doping". Around 0.2 equivalent of oxidant is used to convert PTs (and other conducting polymers) into the optimally conductive state.[citation needed]Thus about one of every five rings is oxidized. Many different oxidants are used. Because of the redox reaction, the conductive form of polythiophene is a salt. An idealized stoichiometry is shown using the oxidant [A]PF6:
- (C4H2S)n+ 1/5n [A]PF6→ (C4H2S)n(PF6)0.2n+ 1/5 nA
In principle, PT can be n-doped using reducing agents, but this approach is rarely practiced.[8]

Upon "p-doping", charged unit called abipolaronis formed. The bipolaron moves as a unit along the polymer chain and is responsible for the macroscopically observed conductivity of the material. Conductivity can approach 1000 S/cm.[9]In comparison, the conductivity ofcopperis approximately 5×105S/cm. Generally, the conductivity of PTs is lower than 1000 S/cm, but high conductivity is not necessary for many applications, e.g. as an antistatic film.
Oxidants
[edit]A variety of reagents have been used to dope PTs.Iodineandbromineproduce highly conductive materials,[9]which are unstable owing to slow evaporation of the halogen.[10]Organic acids,includingtrifluoroacetic acid,propionic acid,andsulfonic acidsproduce PTs with lower conductivities than iodine, but with higher environmental stabilities.[10][11]Oxidative polymerization withferric chloridecan result in doping by residualcatalyst,[12]althoughmatrix-assisted laser desorption/ionizationmass spectrometry(MALDI-MS) studies have shown that poly(3-hexylthiophene)s are also partially halogenated by the residual oxidizing agent.[13]Poly(3-octylthiophene) dissolved intoluenecan be doped by solutions of ferric chloride hexahydrate dissolved inacetonitrile,and can be cast into films with conductivities reaching 1 S/cm.[14]Other, less common p-dopants includegold trichloride[15]andtrifluoromethanesulfonic acid.[16]
Structure and optical properties
[edit]Conjugation length
[edit]The extended π-systems of conjugated PTs produce some of the most interesting properties of these materials—their optical properties. As an approximation, the conjugated backbone can be considered as a real-world example of the "electron-in-a-box" solution to theSchrödinger equation;however, the development of refined models to accurately predictabsorptionandfluorescencespectra of well-defined oligo(thiophene) systems is ongoing.[17]Conjugation relies upon overlap of the π-orbitals of thearomatic rings,which, in turn, requires the thiophene rings to be coplanar.

The number of coplanar rings determines the conjugation length—the longer the conjugation length, the lower the separation between adjacentenergy levels,and the longer the absorption wavelength. Deviation from coplanarity may be permanent, resulting from mislinkages during synthesis or especially bulkyside chains;or temporary, resulting from changes in the environment or binding. This twist in the backbone reduces the conjugation length, and the separation between energy levels is increased. This results in a shorter absorption wavelength.
Determining the maximum effective conjugation length requires the synthesis of regioregular PTs of defined length. The absorption band in the visible region is increasinglyred-shiftedas the conjugation length increases, and the maximum effective conjugation length is calculated as the saturation point of the red-shift. Early studies by ten Hoeveet al.estimated that the effective conjugation extended over 11 repeat units,[18]while later studies increased this estimate to 20 units.[19]Using the absorbance and emission profile of discrete conjugated oligo(3-hexylthiophene)s prepared through polymerization and separation, Lawrenceet al.determined the effective conjugation length of poly(3-hexylthiophene) to be 14 units.[20]The effective conjugation length of polythiophene derivatives depend on the chemical structure of side chains,[21]and thiophene backbones.[22]
The absorption band of poly (3-thiophene acetic acid) in aqueous solutions ofpoly(vinyl alcohol)(PVA) shifts from 480 nm atpH7 to 415 nm at pH 4. This is attributed to formation of a compact coil structure, which can formhydrogen bondswith PVA upon partial deprotonation of the acetic acid group.[23]
Shifts in PT absorption bands due to changes in temperature result from a conformational transition from a coplanar, rodlike structure at lower temperatures to a nonplanar, coiled structure at elevated temperatures. For example, poly(3-(octyloxy)-4-methylthiophene) undergoes a color change from red–violet at 25 °C to pale yellow at 150 °C. Anisosbestic point(a point where the absorbance curves at all temperatures overlap) indicates coexistence between two phases, which may exist on the same chain or on different chains.[24]Not allthermochromicPTs exhibit an isosbestic point: highly regioregular poly(3-alkylthiophene)s (PATs) show a continuous blue-shift with increasing temperature if the side chains are short enough so that they do not melt and interconvert between crystalline and disordered phases at low temperatures.[citation needed]
Optical effects
[edit]The optical properties of PTs can be sensitive to many factors. PTs exhibit absorption shifts due to application of electric potentials (electrochromism),[25]or to introduction ofalkali ions(ionochromism).[26]Soluble PATs exhibit both thermochromism and solvatochromism (seeabove) in chloroform and 2,5-dimethyltetrahydrofuran.[27]
Substituted polythiophenes
[edit]Polythiophene and its oxidized derivatives have poor processing properties. They are insoluble in ordinary solvents and do not melt readily. For example, doped unsubstituted PTs are only soluble in exotic solvents such asarsenic trifluorideandarsenic pentafluoride.[28]Although only poorly processable, "the expected high temperature stability and potentially very high electrical conductivity of PT films (if made) still make it a highly desirable material."[29]Nonetheless, intense interest has focused on soluble polythiophenes, which usually translates to polymers derived from 3-alkylthiophenes, which give the so-called polyalkylthiophenes (PATs).
3-Alkylthiophenes
[edit]Soluble polymers are derivable from 3-substituted thiophenes where the 3-substituent is butyl or longer. Copolymers also are soluble, e.g., poly(3-methylthiophene-'co'-3'-octylthiophene).[29]
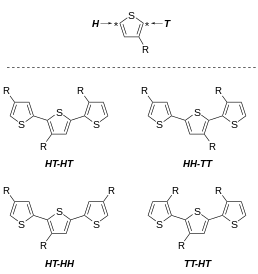
One undesirable feature of 3-alkylthiophenes is the variable regioregularity of the polymer. Focusing on the polymermicrostructureat the dyad level, 3-substituted thiophenes can couple to give any of three dyads:
- 2,5', or head–tail (HT), coupling
- 2,2', or head–head (HH), coupling
- 5,5', or tail–tail (TT), coupling
These three diads can be combined into four distinct triads. The triads are distinguishable byNMR spectroscopy.[30][31]
Regioregularity affects the properties of PTs. A regiorandom copolymer of 3-methylthiophene and 3-butylthiophene possessed a conductivity of 50 S/cm, whereas a more regioregular copolymer with a 2:1 ratio of HT to HH couplings had a higher conductivity of 140 S/cm.[32]Films of regioregular poly(3-(4-octylphenyl)thiophene) (POPT) with greater than 94% HT content possessed conductivities of 4 S/cm, compared with 0.4 S/cm for regioirregular POPT.[33]PATs prepared using Riekezincformed "crystalline, flexible, and bronze-colored films with a metallic luster". On the other hand, the corresponding regiorandom polymers produced "amorphous and orange-colored films".[34]Comparison of the thermochromic properties of the Rieke PATs showed that, while the regioregular polymers showed strong thermochromic effects, the absorbance spectra of the regioirregular polymers did not change significantly at elevated temperatures. Finally, Fluorescence absorption and emission maxima of poly(3-hexylthiophene)s occur at increasingly lower wavelengths (higher energy) with increasing HH dyad content. The difference between absorption and emission maxima, theStokes shift,also increases with HH dyad content, which they attributed to greater relief from conformational strain in the first excited state.[35]
Special substituents
[edit]Water-soluble PT's are represented by sodium poly(3-thiophenealkanesulfonate)s.[36]In addition to conferring water solubility, the pendantsulfonategroups act as counterions, producing self-doped conducting polymers. Substituted PTs with tetheredcarboxylic acidsalso exhibit water solubility.[37][38][39]andurethanes[40]
Thiophenes withchiralsubstituents at the 3 position have been polymerized. Such chiral PTs in principle could be employed for detection or separation of chiral analytes.[41]
Poly(3-(perfluorooctyl)thiophene)s is soluble insupercritical carbon dioxide[42][43]Oligothiophenes capped at both ends with thermally-labile alkyl esters were cast as films from solution, and then heated to remove the solublizing end groups.Atomic force microscopy(AFM) images showed a significant increase in long-range order after heating.[44]
Fluorinated polythiophene yield 7% efficiency in polymer-fullerene solar cells.[45]
PEDOT
[edit]The 3,4-disubstituted thiophene calledethylenedioxythiophene(EDOT) is the precursor to the polymerPEDOT.Regiochemistry is not an issue in since this monomer is symmetrical. PEDOT is found inelectrochromic displays,photovoltaics,electroluminescent displays, printed wiring, and sensors.[46]
Synthesis
[edit]Electrochemical synthesis
[edit]In an electrochemical polymerization, a solution containing thiophene and anelectrolyteproduces a conductive PT film on theanode.[29]Electrochemical polymerization is convenient, since the polymer does not need to be isolated and purified, but it can produce polymers with undesirable Alpha -beta linkages and varying degrees of regioregularity. The stoichiometry of the electropolymerization is:
- n C4H4S → (C4H2S)n+ 2n H++ 2n e−

Thedegree of polymerizationand quality of the resulting polymer depends upon the electrode material, current density, temperature, solvent, electrolyte, presence of water, and monomer concentration.[47]
Electron-donating substituents lower the oxidation potential, whereas electron-withdrawing groups increase the oxidation potential. Thus, 3-methylthiophene polymerizes in acetonitrile and tetrabutylammonium tetrafluoroborate at a potential of about 1.5 V vs.SCE,whereas unsubstituted thiophene requires an additional 0.2 V.Steric hindranceresulting from branching at the α-carbon of a 3-substituted thiophene inhibits polymerization.[48]
In terms of mechanism, oxidation of the thiophene monomer produces aradical cation,which then couple with another monomer to produce a radical cation dimer.
From bromothiophenes
[edit]Chemical synthesis offers two advantages compared with electrochemical synthesis of PTs: a greater selection of monomers, and, using the proper catalysts, the ability to synthesize perfectly regioregular substituted PTs. PTs were chemically synthesized by accident more than a century ago.[49]Chemical syntheses from 2,5-dibromothiophene useKumada couplingand related reactions[50][51]
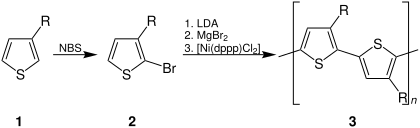
Regioregular PTs have been prepared by lithiation 2-bromo-3-alkylthiophenes usingKumada cross-coupling.[52]This method produces approximately 100% HT–HT couplings, according to NMR spectroscopy analysis of the diads. 2,5-Dibromo-3-alkylthiophene when treated with highly reactive "Rieke zinc" is an alternative method.[53][54]

Routes employing chemical oxidants
[edit]In contrast to methods that require brominated monomers, the oxidative polymerization of thiophenes using ferric chloride proceeds at room temperature. The approach was reported by Sugimotoet al.in 1986.[55]The stoichiometry is analogous to that of electropolymerization.
This method has proven to be extremely popular; antistatic coatings are prepared on a commercial scale using ferric chloride. In addition to ferric chloride, other oxidizing agents have been reported.[29]Slow addition of ferric chloride to the monomer solution produced poly(3-(4-octylphenyl)thiophene)s with approximately 94% H–T content.[33]Precipitation of ferric chloride in situ (in order to maximize the surface area of the catalyst) produced significantly higher yields and monomer conversions than adding monomer directly to crystalline catalyst.[56][57]Higher molecular weights were reported when dry air was bubbled through the reaction mixture during polymerization.[29]ExhaustiveSoxhlet extractionafter polymerization with polar solvents was found to effectively fractionate the polymer and remove residual catalyst before NMR spectroscopy.[30]Using a lower ratio of catalyst to monomer (2:1, rather than 4:1) may increase the regioregularity of poly(3-dodecylthiophene)s.[58]Andreaniet al.reported higher yields of soluble poly(dialkylterthiophene)s incarbon tetrachloriderather than chloroform, which they attributed to the stability of the radical species in carbon tetrachloride.[59]Higher-quality catalyst, added at a slower rate and at reduced temperature, was shown to produce high molecular weight PATs with no insoluble polymer residue.[60]Factorial experimentsindicate that the catalyst/monomer ratio correlated with increased yield of poly(3-octylthiophene). Longer polymerization time also increased the yield.[61]

In terms of mechanism, the oxidative polymerization using ferric chloride, a radical pathway has been proposed. Niemiet al.reported that polymerization was only observed in solvents where the catalyst was either partially or completely insoluble (chloroform,toluene,carbon tetrachloride,pentane,andhexane,and notdiethyl ether,xylene,acetone, orformic acid), and speculated that the polymerization may occur at the surface of solid ferric chloride.[62]However, this is challenged by the fact that the reaction also proceeds in acetonitrile, which FeCl3is soluble in.[63]Quantum mechanical calculationsalso point to a radical mechanism. The mechanism can also be inferred from the regiochemistry of the dimerization of 3-methylthiophene since C2 in [3-methylthiophene]+has the highest spin density.
A carbocation mechanism is inferred from the structure of 3-(4-octylphenyl)thiophene prepared from ferric chloride.[33]
Polymerization of thiophene can be effected by a solution of ferric chloride in acetonitrile. The kinetics of thiophene polymerization also seemed to contradict the predictions of theradical polymerizationmechanism.[63]Barbarellaet al.studied the oligomerization of 3-(alkylsulfanyl)thiophenes, and concluded from their quantum mechanical calculations, and considerations of the enhanced stability of the radical cation when delocalized over a planar conjugated oligomer, that a radical cation mechanism analogous to that generally accepted for electrochemical polymerization was more likely.[64]Given the difficulties of studying a system with a heterogeneous, strongly oxidizing catalyst that produces difficult to characterize rigid-rod polymers, the mechanism of oxidative polymerization is by no means decided. The radical cation mechanism is generally accepted.
Applications
[edit]
As an example of a static application,poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT-PSS)product ( "Clevios P" ) fromHeraeushas been extensively used as an antistatic coating (as packaging materials for electronic components, for example).AGFAcoats 200 m × 10 m ofphotographic filmper year with PEDOT:PSS because of itsantistaticproperties. The thin layer of PEDOT:PSS is virtually transparent and colorless, prevents electrostatic discharges during film rewinding, and reduces dust buildup on the negatives after processing.[46]
Proposed applications
[edit]PEDOT also has been proposed for dynamic applications where a potential is applied to a polymer film. PEDOT-coated windows and mirrors become opaque or reflective upon the application of an electric potential, a manifestation of itselectrochromic properties.[25]Widespread adoption ofelectrochromicwindows promise significant savings inair conditioningcosts.[65]
Another potential application includefield-effect transistors,[66]electroluminescent devices,solar cells,photochemical resists,nonlinear optic devices,[67]batteries,diodes,and chemicalsensors.[68]In general, two categories of applications are proposed for conducting polymers. Static applications rely upon the intrinsicconductivityof the materials, combined with their processing and material properties common to polymeric materials. Dynamic applications utilize changes in the conductive and optical properties, resulting either from application of electric potentials or from environmental stimuli.
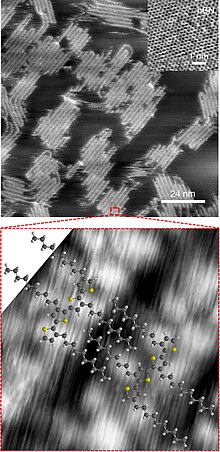
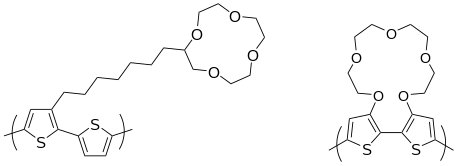
PTs have been touted as sensor elements. In addition tobiosensor applications,PTs can also be functionalized with receptors for detecting metal ions orchiral moleculesas well. PTs with pendantcrown etherfunctionalities exhibit properties that vary with the alkali metal.[69]and main-chain.[26]
Polythiophenes show potential in the treatment ofprion diseases.[70]
Notes
[edit]- ^Strictly speaking, "polythiophene" is a misnomer, since the polymer consists of thienylene (2,5-C4H2S) repeat units. Similarly, thiophene is not a monomer as such.
References
[edit]- ^Arosio, Paolo; Moreno, Margherita; Famulari, Antonino; Raos, Guido; Catellani, Marinella; Valdo Meille, Stefano (2009). "Ordered Stacking of Regioregular Head-to-Tail Polyalkylthiophenes: Insights from the Crystal Structure of Form I′ Poly(3-n-butylthiophene) ".Chem. Mater.21(1): 78–87.doi:10.1021/cm802168e.
- ^Tourillon, G.; Garnier, F. (April 1982). "New electrochemically generated organic conducting polymers".Journal of Electroanalytical Chemistry and Interfacial Electrochemistry.135(1): 173–178.doi:10.1016/0022-0728(82)90015-8.
- ^Österholm, J.-E.; Passiniemi, P.; Isotalo, H.; Stubb, H. (February 1987). "Synthesis and properties of FeCl4-doped polythiophene".Synthetic Metals.18(1–3): 213–218.doi:10.1016/0379-6779(87)90881-2.
- ^Nielsen, Christian B.; McCulloch, Iain (2013). "Recent advances in transistor performance of polythiophenes".Progress in Polymer Science.38(12): 2053–2069.doi:10.1016/j.progpolymsci.2013.05.003.hdl:10044/1/14442.S2CID136757919.
- ^McQuade, D. Tyler; Pullen, Anthony E.; Swager, Timothy M. (2000). "Conjugated Polymer-Based Chemical Sensors".Chemical Reviews.100(7): 2537–74.doi:10.1021/cr9801014.PMID11749295.S2CID4936796.
- ^Mehmood, Umer; Al-Ahmed, Amir; Hussein, Ibnelwaleed A. (2016). "Review on recent advances in polythiophene based photovoltaic devices".Renewable & Sustainable Energy Reviews.57:550–561.Bibcode:2016RSERv..57..550M.doi:10.1016/j.rser.2015.12.177.S2CID101640805.
- ^Kobayashi, M.; Chen, J.; Chung, T.-C.; Moraes, F.; Heeger, A.J.; Wudl, F. (January 1984). "Synthesis and properties of chemically coupled poly(thiophene)".Synthetic Metals.9(1): 77–86.doi:10.1016/0379-6779(84)90044-4.
- ^Mastragostino, M.; Soddu, L. (1990). "Electrochemical characterization of" n "doped polyheterocyclic conducting polymers—I. Polybithiophene".Electrochimica Acta.35(2): 463.doi:10.1016/0013-4686(90)87029-2.
- ^abMcCullough, Richard D.; Tristram-Nagle, Stephanie; Williams, Shawn P.; Lowe, Renae D.; Jayaraman, Manikandan (1993). "Self-orienting head-to-tail poly(3-alkylthiophenes): new insights on structure-property relationships in conducting polymers".Journal of the American Chemical Society.115(11): 4910.doi:10.1021/ja00064a070.S2CID15848137.
- ^abLoponen, M.; Taka, T.; Laakso, J.; Vakiparta, K.; Suuronen, K.; Valkeinen, P.; Osterholm, J. (1991). "Doping and dedoping processes in poly (3-alkylthiophenes)".Synthetic Metals.41(1–2): 479–484.doi:10.1016/0379-6779(91)91111-M.
- ^Bartuš, Ján (1991). "Electrically Conducting Thiophene Polymers".Journal of Macromolecular Science, Part A.28(9): 917–924.doi:10.1080/00222339108054069.
- ^Qiao, X.; Wang, Xianhong; Mo, Zhishen (2001). "The FeCl3-doped poly(3-alkyithiophenes) in solid state ".Synthetic Metals.122(2): 449.doi:10.1016/S0379-6779(00)00587-7.
- ^McCarley, Tracy Donovan; Noble; Dubois, C. J.; McCarley, Robin L. (2001)."MALDI-MS Evaluation of Poly(3-hexylthiophene) Synthesized by Chemical Oxidation with FeCl3".Macromolecules.34(23): 7999.Bibcode:2001MaMol..34.7999M.doi:10.1021/ma002140z.
- ^Heffner, G.; Pearson, D. (1991). "Solution processing of a doped conducting polymer".Synthetic Metals.44(3): 341.doi:10.1016/0379-6779(91)91821-Q.
- ^Abdou, M.S.A.; Holdcroft, Steven (1993). "Oxidation of π-conjugated polymers with gold trichloride: enhanced stability of the electronically conducting state and electroless deposition of Au0".Synthetic Metals.60(2): 93.doi:10.1016/0379-6779(93)91226-R.
- ^Rudge, Andy; Raistrick, Ian; Gottesfeld, Shimshon; Ferraris, John P. (1994). "A study of the electrochemical properties of conducting polymers for application in electrochemical capacitors".Electrochimica Acta.39(2): 273.doi:10.1016/0013-4686(94)80063-4.
- ^Bässler, H. "Electronic Excitation". InElectronic Materials: The Oligomer Approach;Müllen, K.; Wegner, G., Eds.; Wiley-VCH: Weinheim, Germany, 1998,ISBN3-527-29438-4
- ^Ten Hoeve, W.; Wynberg, H.; Havinga, E. E.; Meijer, E. W. (1991)."Substituted.. – undecithiophenes, the longest characterized oligothiophenes".Journal of the American Chemical Society.113(15): 5887.doi:10.1021/ja00015a067.
- ^Meier, H.; Stalmach, U.; Kolshorn, H (September 1997). "Effective conjugation length and UV/vis spectra of oligomers".Acta Polymerica.48(9): 379–384.doi:10.1002/actp.1997.010480905.
- ^Lawrence, Jimmy; Goto, Eisuke; Ren, Jing M.; McDearmon, Brenden; Kim, Dong Sub; Ochiai, Yuto; Clark, Paul G.; Laitar, David; Higashihara, Tomoya (2017-10-04). "A Versatile and Efficient Strategy to Discrete Conjugated Oligomers".Journal of the American Chemical Society.139(39): 13735–13739.doi:10.1021/jacs.7b05299.ISSN0002-7863.PMID28872865.
- ^Nakanishi, Hidetaka; Sumi, Naoto; Aso, Yoshio; Otsubo, Tetsuo (1998). "Synthesis and Properties of the Longest Oligothiophenes: the Icosamer and Heptacosamer".The Journal of Organic Chemistry.63(24): 8632.doi:10.1021/jo981541y.
- ^Izumi, Tsuyoshi; Kobashi, Seiji; Takimiya, Kazuo; Aso, Yoshio; Otsubo, Tetsuo (2003). "Synthesis and Spectroscopic Properties of a Series of β-Blocked Long Oligothiophenes up to the 96-mer: Revaluation of Effective Conjugation Length".Journal of the American Chemical Society.125(18): 5286–7.doi:10.1021/ja034333i.PMID12720435.
- ^De Souza, J.; Pereira, Ernesto C. (2001). "Luminescence of poly(3-thiopheneacetic acid) in alcohols and aqueous solutions of poly(vinyl alcohol)".Synthetic Metals.118(1–3): 167–170.doi:10.1016/S0379-6779(00)00453-7.
- ^Roux, Claudine; Leclerc, Mario (1992). "Rod-to-coil transition in alkoxy-substituted polythiophenes".Macromolecules.25(8): 2141.Bibcode:1992MaMol..25.2141R.doi:10.1021/ma00034a012.
- ^abH. W. Heuer; R. Wehrmann; S. Kirchmeyer (2002). "Electrochromic Window Based on Conducting Poly(3,4-ethylenedioxythiophene)-Poly(styrene sulfonate)".Advanced Functional Materials.12(2): 89.doi:10.1002/1616-3028(20020201)12:2<89::AID-ADFM89>3.0.CO;2-1.
- ^abMarsella, Michael J.; Swager, Timothy M. (1993). "Designing conducting polymer-based sensors: selective ionochromic response in crown ether-containing polythiophenes".Journal of the American Chemical Society.115(25): 12214.doi:10.1021/ja00078a090.
- ^Rughooputh, S. D. D. V.; Hotta, S.; Heeger, A. J.; Wudl, F. (May 1987). "Chromism of soluble polythienylenes".Journal of Polymer Science B.25(5): 1071–1078.Bibcode:1987JPoSB..25.1071R.doi:10.1002/polb.1987.090250508.
- ^Frommer, Jane E. (1986). "Conducting polymer solutions".Accounts of Chemical Research.19(1): 2–9.doi:10.1021/ar00121a001.
- ^abcdeMcCullough, Richard D. (1998). "The Chemistry of Conducting Polythiophenes".Advanced Materials.10(2): 93–116.Bibcode:1998AdM....10...93M.doi:10.1002/(SICI)1521-4095(199801)10:2<93::AID-ADMA93>3.0.CO;2-F.S2CID7147581.
- ^abBarbarella, Giovanna; Bongini, Alessandro; Zambianchi, Massimo (1994). "Regiochemistry and Conformation of Poly(3-hexylthiophene) via the Synthesis and the Spectroscopic Characterization of the Model Configurational Triads".Macromolecules.27(11): 3039.Bibcode:1994MaMol..27.3039B.doi:10.1021/ma00089a022.
- ^Diaz-Quijada, G. A.; et al. (1996). "Regiochemical Analysis of Water Soluble Conductive Polymers: Sodium Poly(ω-(3-thienyl)alkanesulfonates)".Macromolecules.29(16): 5416.Bibcode:1996MaMol..29.5416D.doi:10.1021/ma960126+.
- ^Elsenbaumer, R. L.; Jen, K.-Y.; Miller, G. G.; Eckhardt, H.; Shacklette, L. W.; Jow, R. "Poly (alkylthiophenes) and Poly (substituted heteroaromatic vinylenes): Versatile, Highly Conductive, Processible Polymers with Tunable Properties". InElectronic Properties of Conjugated Polymers(Eds: Kuzmany, H.; Mehring, M.; Roth, S.), Springer, Berlin, 1987,ISBN0-387-18582-8
- ^abcAndersson, M. R.; Selse, D.; Berggren, M.; Jaervinen, H.; Hjertberg, T.; Inganaes, O.; Wennerstroem, O.; Oesterholm, J.-E. (1994). "Regioselective polymerization of 3-(4-octylphenyl)thiophene with FeCl3".Macromolecules.27(22): 6503.Bibcode:1994MaMol..27.6503A.doi:10.1021/ma00100a039.
- ^Chen, Tian-An; Wu, Xiaoming; Rieke, Reuben D. (1995). "Regiocontrolled Synthesis of Poly(3-alkylthiophenes) Mediated by Rieke Zinc: Their Characterization and Solid-State Properties".Journal of the American Chemical Society.117(1): 233–244.doi:10.1021/ja00106a027.
- ^Xu, Bai; Holdcroft, Steven (1993). "Molecular control of luminescence from poly(3-hexylthiophenes)".Macromolecules.26(17): 4457.Bibcode:1993MaMol..26.4457X.doi:10.1021/ma00069a009.
- ^Patil, A. O.; Ikenoue, Y.; Wudl, Fred; Heeger, A. J. (1987). "Water soluble conducting polymers".Journal of the American Chemical Society.109(6): 1858.doi:10.1021/ja00240a044.
- ^Englebienne, Patrick; Weiland, Mich le (1996). "Synthesis of water-soluble carboxylic and acetic acid-substituted poly(thiophenes) and the application of their photochemical properties in homogeneous competitive immunoassays".Chemical Communications(14): 1651.doi:10.1039/cc9960001651.
- ^Kim; Chen, Li; Gong; Osada, Yoshihito (1999). "Titration Behavior and Spectral Transitions of Water-Soluble Polythiophene Carboxylic Acids".Macromolecules.32(12): 3964.Bibcode:1999MaMol..32.3964K.doi:10.1021/ma981848z.
- ^Andersson, M.; Ekeblad, P. O.; Hjertberg, T.; Wennerström, O.; Inganäs, O. (1991). "Polythiophene with a free amino-acid side-chain".Polym. Commun.32:546–548.
- ^Jung, S.; Hwang, D.-H.; Zyung, T.; Kim, W. H.; Chittibabu, K. G.; Tripathy, S. K. (1998). "Temperature dependent photoluminescence and electroluminescence properties of polythiophene with hydrogen bonding side chain".Synthetic Metals.98(2): 107.doi:10.1016/S0379-6779(98)00161-1.
- ^abKane-Maguire, Leon A. P.; Wallace, Gordon G. (2010). "Chiral conducting polymers".Chemical Society Reviews.39(7): 2545–2576.doi:10.1039/b908001p.PMID20567781.
- ^Desimone, J. M.; Guan, Z.; Elsbernd, C. S. (1992). "Synthesis of Fluoropolymers in Supercritical Carbon Dioxide".Science.257(5072): 945–7.Bibcode:1992Sci...257..945D.doi:10.1126/science.257.5072.945.PMID17789638.S2CID35348519.
- ^Li, L.; Counts, K. E.; Kurosawa, S.; Teja, A. S.; Collard, D. M. (2004). "Tuning the Electronic Structure and Solubility of Conjugated Polymers with Perfluoroalkyl Substituents: Poly(3-perfluorooctylthiophene), the First Supercritical CO2-soluble Conjugated Polymer ".Advanced Materials.16(2): 180.Bibcode:2004AdM....16..180L.doi:10.1002/adma.200305333.S2CID97859155.
- ^Murphy, Amanda R.; Fréchet, Jean M. J.; Chang, Paul; Lee, Josephine; Subramanian, Vivek (2004). "Organic Thin Film Transistors from a Soluble Oligothiophene Derivative Containing Thermally Removable Solubilizing Groups".Journal of the American Chemical Society.126(6): 1596–7.doi:10.1021/ja039529x.PMID14871066.S2CID33756974.
- ^Price, Samuel C.; Stuart, Andrew C.; Yang, Liqiang; Zhou, Hua xing; You, Wei (2011)."Fluorine Substituted Conjugated Polymer of Medium Band Gap Yields 7% Efficiency in Polymer−Fullerene Solar Cells".Journal of the American Chemical Society.133(12): 4625–4631.doi:10.1021/ja1112595.PMID21375339.
- ^abGroenendaal, L. B.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J. R. (2000). "Poly(3,4-Ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future".Adv. Mater.12(7): 481–494.Bibcode:2000AdM....12..481G.doi:10.1002/(SICI)1521-4095(200004)12:7<481::AID-ADMA481>3.0.CO;2-C.
- ^Schopf, G.; Koßmehl, G. (1997).Polythiophenes—Electrically Conducting Polymers.Advances in Polymer Science. Vol. 129. pp. 1–166.doi:10.1007/BFb0008700.ISBN978-3-540-61857-7.
{{cite book}}:|journal=ignored (help) - ^Roncali, J.; Garreau, R.; Yassar, A.; Marque, P.; Garnier, F.; Lemaire, M. (1987). "Effects of steric factors on the electrosynthesis and properties of conducting poly(3-alkylthiophenes)".The Journal of Physical Chemistry.91(27): 6706.doi:10.1021/j100311a030.
- ^Meyer, Victor(January–June 1883)."Ueber den Begleiter des Benzols im Steinkohlentheer"[On the companion of benzene in stone coal].Berichte der deutschen chemischen Gesellschaft(in German).16(1): 1465–1478.doi:10.1002/cber.188301601324.
- ^Yamamoto, Takakazu; Sanechika, Kenichi; Yamamoto, Akio (January 1980). "Preparation of thermostable and electric-conducting poly(2,5-thienylene)".Journal of Polymer Science: Polymer Letters Edition.18(1): 9–12.Bibcode:1980JPoSL..18....9Y.doi:10.1002/pol.1980.130180103.
- ^Lin, John W-P.; Dudek, Lesley P. (September 1980)."Synthesis and properties of poly(2,5-thienylene)".Journal of Polymer Science A.18(9): 2869–2873.Bibcode:1980JPoSA..18.2869L.doi:10.1002/pol.1980.170180910.
- ^Chen, Tian An; O'Brien, Richard A.; Rieke, Reuben D. (1993). "Use of highly reactive zinc leads to a new, facile synthesis for polyarylenes".Macromolecules.26(13): 3462.Bibcode:1993MaMol..26.3462C.doi:10.1021/ma00065a036.
- ^Zhu, Lishan; Wehmeyer, Richard M.; Rieke, Reuben D. (1991). "The direct formation of functionalized alkyl(aryl)zinc halides by oxidative addition of highly reactive zinc with organic halides and their reactions with acid chlorides, α,β-unsaturated ketones, and allylic, aryl, and vinyl halides".The Journal of Organic Chemistry.56(4): 1445.doi:10.1021/jo00004a021.
- ^Chen, Tian An; Rieke, Reuben D. (1992). "The first regioregular head-to-tail poly(3-hexylthiophene-2,5-diyl) and a regiorandom isopolymer: nickel versus palladium catalysis of 2(5)-bromo-5(2)-(bromozincio)-3-hexylthiophene polymerization".Journal of the American Chemical Society.114(25): 10087.doi:10.1021/ja00051a066.
- ^Sugimoto, R.; Taketa, S.; Gu, H. B.; Yoshino, K (1986)."Preparation of soluble polythiophene derivatives utilizing transition metal halides as catalysts and their property".Chemistry Express.1(11): 635–638.
- ^Costa Bizzarri, P.; Andreani, Franco; Della Casa, Carlo; Lanzi, Massimiliano; Salatelli, Elisabetta (1995). "Ester-functionalized poly(3-alkylthienylene)s: substituent effects on the polymerization with FeCl3".Synthetic Metals.75(2): 141.doi:10.1016/0379-6779(95)03401-5.
- ^Fraleoni-Morgera, Alessandro; Della-Casa, Carlo; Lanzi, Massimiliano; Costa-Bizzarri, Paolo (2003). "Investigation on Different Procedures in the Oxidative Copolymerization of a Dye-Functionalized Thiophene with 3-Hexylthiophene".Macromolecules.36(23): 8617.Bibcode:2003MaMol..36.8617F.doi:10.1021/ma0348730.
- ^Qiao, X.; Wang, Xianhong; Zhao, Xiao gian g; Liu, Jian; Mo, Zhishen (2000). "Poly(3-dodecylthiophenes) polymerized with different amounts of catalyst".Synthetic Metals.114(3): 261.doi:10.1016/S0379-6779(00)00233-2.
- ^Andreani, F.; Salatelli, E.; Lanzi, M. (February 1996). "Novel poly(3,3" – and 3',4'-dialkyl- 2,2':5',2 "– terthiophene)s by chemical oxidative synthesis: evidence for a new step towards the optimization of this process".Polymer.37(4): 661–665.doi:10.1016/0032-3861(96)83153-3.
- ^Gallazzi, M.; Bertarelli, C.; Montoneri, E. (2002). "Critical parameters for product quality and yield in the polymerisation of 3,3" -didodecyl-2,2′:5′,2 "-terthiophene".Synthetic Metals.128(1): 91–95.doi:10.1016/S0379-6779(01)00665-8.
- ^Laakso, J.; Jarvinen, H.; Skagerberg, B. (1993). "Recent developments in the polymerization of 3-alkylthiophenes".Synthetic Metals.55(2–3): 1204.doi:10.1016/0379-6779(93)90225-L.
- ^Niemi, V. M.; Knuuttila, P.; Österholm, J. E.; Korvola, J. (1992). "Polymerization of 3-alkylthiophenes with ferric chloride".Polymer.33(7): 1559–1562.doi:10.1016/0032-3861(92)90138-M..
- ^abOlinga, T.; François, B. (1995). "Kinetics of polymerization of thiophene by FeCl3in choloroform and acetonitrile ".Synthetic Metals.69(1–3): 297–298.doi:10.1016/0379-6779(94)02457-A.
- ^Barbarella, Giovanna; Zambianchi, Massimo; Di Toro, Rosanna; Colonna, Martino; Iarossi, Dario; Goldoni, Francesca; Bongini, Alessandro (1996). "Regioselective Oligomerization of 3-(Alkylsulfanyl)thiophenes with Ferric Chloride".The Journal of Organic Chemistry.61(23): 8285–8292.doi:10.1021/jo960982j.PMID11667817.
- ^Rosseinsky, D. R.; Mortimer, R. J. (2001). "Electrochromic Systems and the Prospects for Devices".Advanced Materials.13(11): 783.Bibcode:2001AdM....13..783R.doi:10.1002/1521-4095(200106)13:11<783::AID-ADMA783>3.0.CO;2-D.S2CID137731242.
- ^Garnier, F. "Field-Effect Transistors Based on Conjugated Materials". InElectronic Materials: The Oligomer Approach(Eds: Müllen, K.; Wegner, G.), Wiley-VCH, Weinheim, 1998,ISBN3-527-29438-4
- ^Harrison, M. G.; Friend, R. H. "Optical Applications". InElectronic Materials: The Oligomer Approach(Eds: Müllen, K.; Wegner, G.), Wiley-VCH, Weinheim, 1998,ISBN3-527-29438-4
- ^Martina, V; Ionescu, K.; Pigani, L; Terzi, F; Ulrici, A.; Zanardi, C.; Seeber, R (March 2007). "Development of an electronic tongue based on a PEDOT-modified voltammetric sensor".Analytical and Bioanalytical Chemistry.387(6): 2101–2110.doi:10.1007/s00216-006-1102-1.PMID17235499.S2CID12701566.
- ^Bäuerle, Peter; Scheib, Stefan (1993). "Molecular recognition of alkali-ions by crown-ether-functionalized poly(alkylthiophenes)".Advanced Materials.5(11): 848.Bibcode:1993AdM.....5..848B.doi:10.1002/adma.19930051113.
- ^Margalith, Ilan; Suter, Carlo; Ballmer, Boris; Schwarz, Petra (2012)."Polythiophenes Inhibit Prion Propagation by Stabilizing Prion Protein (PrP) Aggregates".The Journal of Biological Chemistry.287(23): 18872–87.doi:10.1074/jbc.M112.355958.PMC3365923.PMID22493452.
Further reading
[edit]- Handbook of Conducting Polymers(Eds: T. A. Skotheim, R. L. Elsenbaumer, J. R. Reynolds), Marcel Dekker, New York, 1998.ISBN0-8247-0050-3
- G. Schopf, G. Koßmehl,Polythiophenes: Electrically Conductive Polymers,Springer, Berlin, 1997,ISBN3-540-61483-4;ISBN0-387-61483-4
- Synthetic Metals(journal). ISSN 0379-6779
- Street, G. B.; Clarke, T. C. (1981). "Conducting Polymers: A Review of Recent Work".IBM J. Res. Dev.25(1): 51–57.doi:10.1147/rd.251.0051.
- Roncali, Jean (1992). "Conjugated poly(thiophenes): synthesis, functionalization, and applications".Chemical Reviews.92(4): 711–738.doi:10.1021/cr00012a009.
- Roncali, Jean (1997). "Synthetic Principles for Bandgap Control in Linear π-Conjugated Systems".Chemical Reviews.97(1): 173–206.doi:10.1021/cr950257t.PMID11848868.
- Reddinger, J. L.; Reynolds, J. R. (1999).Molecular Engineering of p-Conjugated Polymers.Advances in Polymer Science. Vol. 145. pp. 57–122.doi:10.1007/3-540-70733-6_2.ISBN978-3-540-65210-6.

