O-Acetylpsilocin
This articleneeds additional citations forverification.(March 2011) |
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Psilacetin; 4-Acetoxy-N,N-dimethyltryptamine; 4-Acetoxy-DMT; 4-AcO-DMT; Synthetic shrooms; 3-(2'-Dimethylaminoethyl)-4-acetoxyindole[1] |
| Routes of administration | Oral,intravenous,intranasal,rectal |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
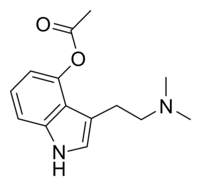
| Formula | C14H18N2O2 |
| Molar mass | 246.310g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 172 to 173 °C (342 to 343 °F) |
| |
| |
| (verify) | |
Psilacetin,also known asO-acetylpsilocinor as4-acetoxy-N,N-dimethyltryptamine(4-acetoxy-DMTor4-AcO-DMT), is asemi-syntheticserotonergic psychedelicdrugthat has been suggested byDavid Nicholsto be a potentially useful alternative topsilocybinfor pharmacological studies, as they are both believed to beprodrugsofpsilocin.[2][3]However, some users report thatO-acetylpsilocin's subjective effects differ from those of psilocybin and psilocin.[4][5]Additionally, some users prefer 4-AcO-DMT to naturalpsilocybin mushroomsdue to feeling fewer adverse side effects such as nausea and heavy body load, which are more frequently reported in experiences involving natural mushrooms.[6]It is theacetylatedform of thepsilocybin mushroomalkaloidpsilocinand is a lowerhomologof4-AcO-MET,4-AcO-DET,4-AcO-MiPTand4-AcO-DiPT.
In 2024, it was confirmed that psilacetin is aprodrugofpsilocin.[7]
Pharmacology
[edit]- Seepsilocinfor more details.
In the body,O-acetylpsilocin isdeacetylatedto psilocin bydeacetylases/acetyltransferasesduringfirst pass metabolism[citation needed]and during subsequent passes through the liver (evident as psilacetin is also active via parenteral routes of ingestion).
Claims of subjective differences in effects between the acetylated and non-acetylated forms of psilocin vary:[4]some users report thatO-acetylpsilocin lasts slightly longer, whilst others report that it lasts for a considerably shorter time. Many users report lessbody loadand nausea compared with psilocin. Some users find that the visual effects produced byO-acetylpsilocin more closely resemble those produced byDMTthan those produced by psilocin or psilocybin. These differences could be possible if psilacetin is psychoactive in itself and not merely as a prodrug. Despite this, there have been no controlled clinical studies to distinguish among the phenomenological effects of psilacetin, psilocin, and psilocybin.

Chemistry
[edit]O-Acetylpsilocin can be obtained byacetylationof psilocin underalkalineor stronglyacidicconditions. It is, therefore, asyntheticcompound. It is believed to be aprodrugof psilocin; however, speculation exists that psilacetin itself also may be psychoactive.O-Acetylpsilocin is more resistant than psilocin to oxidation under basic conditions due to its acetoxy group. WhileO-acetylpsilocin is not well researched (sometimes viewed negatively as aresearch chemical,as opposed to psilocin and psilocybin), it is not as difficult as psilocybin to synthesize. Due to their similar proposed mechanisms of action, this factor may provide further support for the proposition thatO-acetylpsilocin might serve as an appropriate substitute for psilocybin in research of the application of psychedelic compounds in medicine.[2]
Given enough time in unfavorable conditions,O-acetylpsilocin can sometimes turn into a degraded form which is brown in color and can even progress into a brown/black tar-like substance. Researchers hypothesize this is a polymerization reaction and is said to have no effect on the potency of the substance. PreliminaryGCMSanalysis of the closely related homolog 4-acetoxy-DET suggests that this degraded form ofO-acetylpsilocin consists mainly of the hydroxy form of the parent molecule.[8]
History
[edit]O-Acetylpsilocin (psilacetin) and several other esters of psilocin were patented on January 16, 1963, bySandozLtd viaAlbert Hofmann& Franz Troxler.[1][9]Despite this, psilacetin remains a psychedelic compound with a limited history of use. It is theorized to be aprodrugof psilocin, as is psilocybin, which occurs naturally in many species of psychedelic mushrooms. This is because the aromatic acetyl moiety on the 4th position of the indole ring system is subject to deacetylation in acidic conditions such as those found in the stomach.[10]Psilacetin isO-acetylated psilocin, whereas psilocybin isO-phosphorylated.
Society and culture
[edit]Legal status
[edit]This articleneeds additional citations forverification.(March 2011) |
Australia
[edit]O-Acetylpsilocin can be considered an analog ofpsilocinmaking it a Schedule 9 prohibited substance in Australia under thePoisons Standard(October 2015).[11]A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[11]
United States
[edit]O-Acetylpsilocin is ambiguously legal for use as a lab reagent or research chemical; however, it is an acetate ester of psilocin, meaning it would be considered akin to a Schedule I Controlled Substance under theFederal Analogue Actif sold for human consumption.
O-Acetylpsilocin is listed (often under 4-Aco-DMT) as a controlled substance at the state level in multiple states in the USA, includingAlabamawhich has made it a schedule I at the state level on March 18th, 2014, along with several other tryptamine analogs.[12]
United Kingdom
[edit]O-Acetylpsilocin, being an ester of psilocin, is aClass A drugin the UK under theMisuse of Drugs Act 1971.[13]
Czech Republic
[edit]O-Acetylpsilocin is prohibited in Czech republic except strictly limited research and therapeutical purposes.[14]
Italy
[edit]O-Acetylpsilocin is illegal in Italy as it is anesterof a prohibited substance.
Sweden
[edit]The Riksdagadded 4-AcO-DMT toNarcotic Drugs Punishments Actunderswedish schedule I("substances, plant materials and fungi which normally do not have medical use") as of January 25, 2017, published byMedical Products Agency (MPA)in regulationHSLF-FS 2017:1listed as "4-acetoxi-N,N-dimetyltryptamin ".[15]
Israel
[edit]O-Acetylpsilocin is technically illegal in Israel as of being a derivative of DMT.
See also
[edit]References
[edit]- ^abUS patent 3075992,Hofmann A, Troxler F, "Esters of indoles", assigned to Sandoz Ltd.
- ^abNichols D, Fescas S (1999)."Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin"(PDF).Synthesis.1999(6): 935–938.CiteSeerX10.1.1.690.8071.doi:10.1055/s-1999-3490.S2CID32044725.Archived(PDF)from the original on 17 February 2012.Retrieved17 January2012.
- ^Bauer BE (2019-09-18)."The State of the Art of Psilacetin (4-AcO-DMT)".Psychedelic Science Review.Retrieved2021-02-13.
- ^ab"4-AcO-DMT (also 4-acetoxy-N,N-dimethyltryptamine): Erowid Exp: Main Index".erowid.org.Archivedfrom the original on 2010-07-28.
- ^Janikian M (2020-05-26)."The Complete Guide: 4-AcO-DMT a.k.a. Synthetic Shrooms".DoubleBlind Mag.
- ^Palamar JJ, Acosta P (January 2020)."A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines".Human Psychopharmacology.35(1): e2719.doi:10.1002/hup.2719.PMC6995261.PMID31909513.
- ^Jones NT, Wagner L, Hahn MC, Scarlett CO, Wenthur CJ (2023)."In vivo validation of psilacetin as a prodrug yielding modestly lower peripheral psilocin exposure than psilocybin".Front Psychiatry.14:1303365.doi:10.3389/fpsyt.2023.1303365.PMC10804612.PMID38264637.
- ^"Erowid 4-Acetoxy-DET Vaults: 4-Acetoxy-DET / Ethacetin Degradation".erowid.org.Retrieved10 September2023.
- ^US 3075992
- ^Staněk J, Černá MJ (January 1963). "Acidic deacetylation of sugar acetates".Tetrahedron Letters.4(1): 35–7.doi:10.1016/S0040-4039(01)90572-6.
- ^ab"Poisons Standard October 2015".Federal Register of Legislation.Australian Government. 30 September 2015.Archivedfrom the original on 2016-01-19.Retrieved2016-01-06.
- ^"Controlled Substances List"(PDF).Alabama State Board of Health.22 February 2024. p. 50. Archived fromthe original(PDF)on 8 August 2024.Retrieved18 August2024.
- ^Misuse of Drugs Act 1971(Schedule 2 Part I). 1971.
- ^"Government regulation of the list of the addictive substances".Federal Register of Legislation.Czech Government.
- ^"Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika"[Regulations on changes in the Swedish Medicines Agency's regulations (LVFS 2011:10) on lists of narcotics](PDF)(in Swedish).Archived(PDF)from the original on 2017-10-31.Retrieved2017-04-21.
External links
[edit]- 4-AcO-DMT information at IsomerDesign
- 4-AcO-DMTat PsychonautWiki
- Erowid 4-Acetoxy-DMT Vault
- "Esters of Indoles" US Patent # 3,075,992- Awarded toSandozLtd. (viaAlbert Hofmann& Franz Troxler) on January 29, 1963.
