Relapse
Ininternal medicine,relapseorrecidivismis a recurrence of a past (typically medical) condition. For example,multiple sclerosisandmalariaoften exhibit peaks of activity and sometimes very long periods of dormancy, followed by relapse orrecrudescence.
Inpsychiatry,relapseorreinstatement of drug-seeking behavior,is the recurrence of pathological drug use, self harm or other symptoms after a period of recovery. Relapse is often observed in individuals who have developed adrug addictionor a form ofdrug dependence,as well as those who have amental disorder.
Risk factors
[edit]Dopamine D2 receptor availability
[edit]The availability of thedopamine receptor D2plays a role inself-administrationand thereinforcing effectsofcocaineand otherstimulants.The D2 receptor availability has aninverse relationshipto the vulnerability of reinforcing effects of thedrug.With the D2 receptors becoming limited, the user becomes more susceptible to the reinforcing effects of cocaine. It is currently unknown if apredispositionto low D2 receptor availability is possible; however, most studies support the idea that changes in D2 receptor availability are aresult,rather than aprecursor,of cocaine use. It has also been noted that D2 receptors may return to the level existing prior to drug exposure during long periods ofabstinence,a fact which may have implications in relapsetreatment.[1]
Social hierarchy
[edit]Social interactions,such as the formation oflinear dominance hierarchies,also play a role in vulnerability to substance use.Animal studiessuggest that there exists a difference in D2 receptor availability betweendominantand subordinate animals within asocial hierarchyas well as a difference in the function of cocaine toreinforceself-administration in these animal groups. Socially dominant animals exhibit higher availability of D2 receptors and fail to maintain self-administration.[2]
Triggers
[edit]Drug taking and relapse are heavily influenced by a number of factors including thepharmacokinetics,dose,andneurochemistryof the drug itself as well as the drug taker’senvironmentand drug-related history. Reinstatement of drug use after a period of non-use or abstinence is typically initiated by one or a combination of the three main triggers:stress,re-exposure to the drug or drug-priming, and environmentalcues.These factors may induce aneurochemicalresponse in the drug taker that mimics the drug and thus triggers reinstatement.[3]These cues may lead to a strong desire or intention to use the drug, a feeling termedcravingbyAbraham Wiklerin 1948. The propensity for craving is heavily influenced by all three triggers to relapse and is now an accepted hallmark ofsubstance dependence.[4]Stress is one of the most powerful stimuli for reinstating drug use because stress cues stimulate craving and drug-seeking behavior duringabstinence.Stress-induced craving is also predictive of time to relapse. Comparably, addicted individuals show an increased susceptibility tostressorsthan do non-addicted controls. Examples of stressors that may induce reinstatement include emotions offear,sadness,oranger,a physical stressor such as a footshock or elevated sound level, or a social event.[5]Drug-priming is exposing the abstinent user to the addictive substances, which will induce reinstatement of the drug-seeking behavior and drug self-administration.[6]Stimuli that have a pre-existing association with a given drug or with use of that drug can trigger both craving and reinstatement. These cues include any items, places, or people associated with the drug.[7]
Treatment
[edit]Relapse treatment is somewhat of amisnomerbecause relapse itself is a treatment failure; however there exist three main approaches that are currently used to reduce the likelihood of drug relapse. These includepharmacotherapy,cognitive behavioral techniques,andcontingency management.The main goals of treating substance dependence and preventing relapse are to identify theneedsthat were previously met by use of the drug and to develop the skills needed to meet those needs in an alternative way.[7]
Pharmacotherapy
[edit]- Related article:Drug rehabilitation
Variousmedicationsare used to stabilize an addicted user, reduce the initial drug use, and prevent reinstatement of the drug. Medications can normalize the long-term changes that occur in thebrainandnervous systemas a result of prolonged drug use. This method of therapy is complex and multi-faceted because the brain target for the desire to use the drug may be different from the target induced by the drug itself.[8]The availability of variousneurotransmitter receptors,such as thedopamine receptor D2,and changes in themedial prefrontal cortexare prominent targets for pharmacotherapy to prevent relapse because they are heavily linked to drug-induced, stress-induced, and cue-induced relapse. Receptor recovery can beupregulatedby administration ofreceptor antagonists,while pharmacotherapeutic treatments forneruoadaptationsin the medial prefrontal cortex are still relatively ineffective due to lacking knowledge of theseadaptationson themolecularandcellularlevel.[1][9]
Cognitive behavioral techniques
[edit]The various behavioral approaches to treating relapse focus on the precursors and consequences of drug-taking and reinstatement. Cognitive-behavioral techniques (CBT) incorporatePavlovian conditioningandoperant conditioning,characterized bypositive reinforcementandnegative reinforcement,in order to alter thecognitions,thoughts,andemotionsassociated with drug-taking behavior. A main approach of CBT is cue exposure, during which the abstinent user is repeatedly exposed to the most salient triggers without exposure to the substance in hopes that the substance will gradually lose the ability to induce drug-seeking behavior. This approach is likely to reduce the severity of a relapse than to prevent one from occurring altogether. Another method teaches addicts basiccoping mechanismsto avoid using theillicit drug.It is important to address any deficits in coping skills, to identify the needs that likely inducedrug-seeking,and to develop another way to meet them.[10]
Relapse prevention
[edit]Relapse preventionattempts to group the factors that contribute to relapse into two broad categories: immediate determinants and covert antecedents. Immediate determinants are the environmental and emotional situations that are associated with relapse, including high-risk situations that threaten an individual’s sense of control,coping strategies,andoutcome expectancies.Covert antecedents, which are less obvious factors influencing relapse, include lifestyle factors such as stress level and balance, and urges andcravings.The relapse prevention model teaches addicts to anticipate relapse by recognizing and coping with various immediate determinants and covert antecedents. The RP model shows the greatest success with treatment ofalcoholismbut it has not been proven superior to other treatment options.[7][10]Relapse may also be more likely to occur during certain times, such as the holiday season when stress levels are typically higher.[11]So, emphasizing relapse prevention strategies during these times is ideal.
Contingency management
[edit]In contrast to the behavioral approaches above,contingency managementconcentrates on the consequences of drug use as opposed to its precursors. Addict behavior isreinforced,by reward orpunishment,based on ability to remainabstinent.A common example of contingency management is atokenorvoucher system,in which abstinence is rewarded with tokens or vouchers that individuals can redeem for various retail items.[12]
Animal models
[edit]There are vastethical limitationsindrug addictionresearch because humans cannot be allowed to self-administer drugs for the purpose of being studied.[8]However, much can be learned about drugs and the neurobiology of drug taking by the examination of laboratory animals.[13]Most studies are performed on rodents ornon-human primateswith the latter being most comparable to humans inpharmacokinetics,anatomyof theprefrontal cortex,social behavior,andlife span.[14]Other advantages to studying relapse in non-human primates include the ability of the animal to reinstateself-administration,and to learn complex behaviors in order to obtain the drug.[8]Animal studies have shown that a reduction in negative withdrawal symptoms is not necessary to maintain drug taking in laboratory animals; the key to these studies is operant conditioning and reinforcement.[3]
Protocols
[edit]Self-administration
[edit]To self-administer the drug of interest the animal is implanted with an intravenouscatheterand seated in a primate chair equipped with a response lever. The animal is seated in a ventilated chamber and trained on a schedule of drug self-administration. In many studies the self-administration task begins with presentation of a stimulus light (located near the response panel) that may change colors or turn off upon completion of the operant task. The change in visual stimulus is accompanied by an injection of the given drug through the implanted catheter. This schedule is maintained until the animals learn the task.[15]
Extinction
[edit]Extinctionin non-human primates is analogous, with some limitations, to abstinence in humans. In order to extinguish drug-seeking behavior the drug is substituted with asalinesolution. When the animal performs the task it has been trained to perform it is no longer reinforced with an injection of the drug. The visual stimulus associated with the drug and completion of the task is also removed. The extinction sessions are continued until the animal ceases the drug-seeking behavior by pressing the lever.[16]
Reinstatement
[edit]After the animal’s drug-seeking behavior is extinguished, a stimulus is presented to promote the reinstatement of that same drug-seeking behavior (i.e., relapse). For example, if the animal receives an injection of the drug in question it will likely begin working on the operant task for which it was previously reinforced.[6]The stimulus may be the drug itself, the visual stimulus that was initially paired with the drug intake, or a stressor such as anacoustic startleor foot shock.[15]However, the stimulus used to trigger reinstatement can influence the psychological processes involved.[17][18]
Neuroimaging
[edit]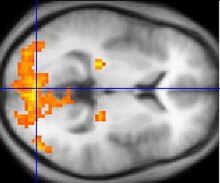
Neuroimaginghas contributed to the identification of theneuralcomponents involved in drug reinstatement as well as drug-taking determinants such as thepharmokinetics,neurochemistry,anddoseof the drug. The neuroimaging techniques used in non-human primates includepositron emission tomography(PET), which usesradiolabeledligandtracers to measure neurochemistryin vivoandsingle-photon emission computed tomography(SPECT).[3]Functional magnetic resonance imaging(fMRI) is widely used in human subjects because it has much higher resolution and eliminates exposure toradiation.[14]
Limitations
[edit]Although the reinstatementprotocolsare used frequently inlaboratorysettings there are some limitations to thevalidityof the procedures as a model ofcravingand relapse in humans. The primary limiting factor is that in humans, relapse rarely follows the strictextinctionof drug-seeking behavior. Additionally, human self-reports show that drug-associatedstimuliplay a lesser role in craving in humans than in the laboratory models. Thevalidityof the model can be examined in three ways:formal equivalence,correlational models, and functional equivalence. There is moderate formal equivalence, orface validity,meaning that the model somewhat resembles relapse as it occurs outside of the laboratory setting; however, there is little face validity for the procedures as a model of craving. Thepredictive validity,which is assessed by correlational models, has yet to be determined for the procedures. There is sound functional equivalence for the model, which suggests that relapse in the laboratory is reasonably similar to that in nature. Further research into other manipulations or reinforcements that could limit drug-taking in non-human primates would be extremely beneficial to the field.[19]
Differences between sexes
[edit]There exists a higher rate of relapse, shorter periods ofabstinence,and higher responsiveness to drug-related cues in women as compared to men. One study suggests that theovarian hormones,estradiolandprogesterone,that exist in females at fluctuating levels throughout themenstrual cycle(orestrous cyclein rodents), play a significant role in drug-primed relapse. There is a marked increase in progesterone levels and a decrease in estradiol levels during theluteal phase.Anxiety,irritability, anddepression,three symptoms of bothwithdrawaland the human menstrual cycle, are most severe in the luteal phase. Symptoms of withdrawal not associated with the cycle, such as hunger, are also enhanced during the luteal phase, which suggests the role of estradiol and progesterone in enhancing symptoms above the naturally occurring level of the menstrual cycle. The symptoms of craving also increase during the luteal phase in humans (it is important to note that the opposite result occurs in female subjects with cocaine addiction suggesting that cyclic changes may be specific for different addictive substances). Further, the drug-primed response is decreased during the luteal phase suggesting a time in the cycle during which the urge to continue use may be reduced. These findings implicate a cyclic, hormone-based timing for quitting an addictive substance and preparing for magnified symptoms of withdrawal or susceptibility to relapse.[20][21]
See also
[edit]References
[edit]- ^abCzoty PW, Gage HD, Nader MA (December 2005). "PET imaging of striatal dopamine D2 receptors in nonhuman primates: increases in availability produced by chronic raclopride treatment".Synapse.58(4): 215–9.doi:10.1002/syn.20200.PMID16206180.
- ^Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA (July 2004). "Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine".Psychopharmacology.174(3): 381–8.doi:10.1007/s00213-003-1752-z.PMID14767632.
- ^abcMurnane KS, Howell LL (July 2011)."Neuroimaging and drug taking in primates".Psychopharmacology.216(2): 153–71.doi:10.1007/s00213-011-2222-7.eISSN1432-2072.ISSN0033-3158.OCLC2409222.PMC3232674.PMID21360099.
- ^Wikler A(November 1948). "Recent progress in research on the neurophysiologic basis of morphine addiction".Am J Psychiatry.105(5): 329–38.doi:10.1176/ajp.105.5.329.PMID18890902.
- ^Breese GR, Sinha R, Heilig M (February 2011)."Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse".Pharmacol. Ther.129(2): 149–71.doi:10.1016/j.pharmthera.2010.09.007.PMC3026093.PMID20951730.
- ^abMcClung J, Fantegrossi W, Howell LL (May 2010)."Reinstatement of extinguished amphetamine self-administration by 3,4-methylenedioxymethamphetamine (MDMA) and its enantiomers in rhesus monkeys".Psychopharmacology.210(1): 75–83.doi:10.1007/s00213-010-1818-7.eISSN1432-2072.ISSN0033-3158.OCLC2409222.PMC2862592.PMID20309529.
- ^abcLarimer ME, Palmer RS, Marlatt GA (1999). "Relapse prevention. An overview of Marlatt's cognitive-behavioral model".Alcohol Res Health.23(2): 151–60.PMID10890810.
- ^abcNader MA, Czoty PW (August 2005). "PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation".Am J Psychiatry.162(8): 1473–82.doi:10.1176/appi.ajp.162.8.1473.PMID16055768.
- ^Van den Oever MC, Spijker S, Smit AB, De Vries TJ (November 2010). "Prefrontal cortex plasticity mechanisms in drug seeking and relapse".Neurosci Biobehav Rev.35(2): 276–84.doi:10.1016/j.neubiorev.2009.11.016.PMID19932711.
- ^abKadden RM (2002-09-10)."Cognitive-Behavior Therapy for Substance Dependence: Coping Skills Training"(PDF).Behavioral Health Recovery Management, University of Chicago. Archived fromthe original(pdf)on 2012-01-05.Retrieved2011-12-03.
- ^Homes, Eudaimonia Recovery (2021-12-20)."Why Is Relapse More Common During the Holiday Season?".Eudaimonia Sober Living Homes.Retrieved2021-12-24.
- ^Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST (February 2006). "A meta-analysis of voucher-based reinforcement therapy for substance use disorders".Addiction.101(2): 192–203.doi:10.1111/j.1360-0443.2006.01311.x.PMID16445548.
- ^Howell LL, Votaw JR, Goodman MM, Lindsey KP (February 2010)."Cortical activation during cocaine use and extinction in rhesus monkeys".Psychopharmacology.208(2): 191–9.doi:10.1007/s00213-009-1720-3.eISSN1432-2072.ISSN0033-3158.OCLC2409222.PMC2819208.PMID19924404.
- ^abHowell LL, Murnane KS (May 2011)."Nonhuman primate positron emission tomography neuroimaging in drug abuse research".J. Pharmacol. Exp. Ther.337(2): 324–34.doi:10.1124/jpet.108.136689.PMC3083112.PMID21317354.
- ^abKirkland Henry P, Davis M, Howell LL (August 2009)."Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys".Psychopharmacology.205(2): 237–47.doi:10.1007/s00213-009-1534-3.eISSN1432-2072.ISSN0033-3158.OCLC2409222.PMC2796974.PMID19365621.
- ^Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL (June 2010)."Dopamine transporter-related effects of modafinil in rhesus monkeys".Psychopharmacology.210(3): 439–48.doi:10.1007/s00213-010-1839-2.PMC2874656.PMID20386883.
- ^Lay, Belinda Po Pyn; Khoo, Shaun Yon-Seng (23 February 2021)."Associative processes in addiction relapse models: A review of their Pavlovian and instrumental mechanisms, history, and terminology".Neuroanatomy and Behaviour.3:e18.doi:10.35430/nab.2021.e18.
- ^Bouton, Mark E.; Maren, Stephen; McNally, Gavan P. (1 April 2021)."Behavioral and neurobiological mechanisms of pavlovian and instrumental extinction learning".Physiological Reviews.101(2): 611–681.doi:10.1152/physrev.00016.2020.PMC8428921.
- ^Katz JL, Higgins ST (July 2003). "The validity of the reinstatement model of craving and relapse to drug use".Psychopharmacology.168(1–2): 21–30.doi:10.1007/s00213-003-1441-y.PMID12695875.
- ^Hudson A, Stamp JA (January 2011). "Ovarian hormones and propensity to drug relapse: a review".Neurosci Biobehav Rev.35(3): 427–36.doi:10.1016/j.neubiorev.2010.05.001.PMID20488201.
- ^Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA (February 2009)."Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys".Neuropsychopharmacology.34(3): 548–54.doi:10.1038/npp.2008.3.eISSN1740-634X.ISSN0893-133X.OCLC815994337.PMID18256593.
