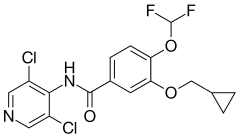
Roflumilast
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daxas, Daliresp, Zoryve, others |
| AHFS/Drugs | Monograph |
| MedlinePlus | a611034 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth,topical |
| Drug class | PDE4 inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | 79%[4][3][8][9] |
| Protein binding | 99%[4][3][8][9] |
| Metabolism | HepaticviaCYP1A2&CYP3A4[4][3][8][9] |
| Eliminationhalf-life | 17 hours (30 hours [active metabolite])[4][3][8][9] |
| Excretion | Urine (70%)[4][3][8][9] |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.210.960 |
| Chemical and physical data | |
| Formula | C17H14Cl2F2N2O3 |
| Molar mass | 403.21g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Roflumilast,sold under the brand nameDaxasamong others, is a medication used for the treatment ofchronic obstructive pulmonary disease,[4]plaque psoriasis,[5]seborrheic dermatitis,[6]andatopic dermatitis.[5]It acts as a selective, long-actinginhibitor of the enzyme phosphodiesterase-4(PDE-4).[10]It hasanti-inflammatoryeffects.[10][11][12]
It was approved for medical use in the European Union in 2010,[7]in the United States in 2011,[4]and in Canada in 2017.[1]It is available as ageneric medication.[13]
Medical uses
[edit]Roflumilast isindicatedfor the treatment of severe chronic obstructive pulmonary disease (COPD),[4]plaquepsoriasis,[5][14]seborrheic dermatitis,[6]and atopic dermatitis,[5][15]
It is used in the prevention of exacerbations (lung attacks) in severe chronic obstructive pulmonary disease (COPD).[3][4][7][8][9]
Adverse effects
[edit]Common (1–10% incidence) adverse effects include diarrhea, weight loss, nausea, headache, insomnia, decreased appetite, abdominal pain, rhinitis, sinusitis, urinary tract infection, and depression.[4][3][8][9][16]
Society and culture
[edit]Legal status
[edit]In June 2010, it was approved in the European Union for severe COPD associated with chronic bronchitis.[7][17]In February 2011, it gained FDA approval in the United States for reducing COPD exacerbations.[18][19]
References
[edit]- ^ab"Daxas Product information".Health Canada.20 July 2017.Retrieved13 July2024.
- ^"Zoryve Product information".Health Canada.7 November 2023.Retrieved13 July2024.
- ^abcdefgh"Daxas 250 micrograms tablets - Summary of Product Characteristics (SmPC)".(emc).11 June 2020.Archivedfrom the original on 19 September 2020.Retrieved28 September2020.
- ^abcdefghijk"Daliresp- roflumilast tablet".DailyMed.12 March 2020.Archivedfrom the original on 25 March 2021.Retrieved28 September2020.
- ^abcde"Zoryve- roflumilast cream".DailyMed.16 August 2022.Archivedfrom the original on 21 January 2023.Retrieved21 January2023.
- ^abc"Zoryve- roflumilast aerosol, foam".DailyMed.20 December 2023.Retrieved13 July2024.
- ^abcd"Daxas EPAR".European Medicines Agency.17 September 2018.Archivedfrom the original on 12 August 2020.Retrieved28 September2020.
- ^abcdefg"Daliresp: EPAR - Product Information"(PDF).European Medicines Agency.Takeda GmbH. 26 September 2013.Archived(PDF)from the original on 26 June 2016.Retrieved18 November2013.
- ^abcdefg"roflumilast (Rx) - Daliresp".Medscape Reference.WebMD.Archivedfrom the original on 12 September 2017.Retrieved18 November2013.
- ^abBoswell-Smith V, Spina D (2007)."PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast".International Journal of Chronic Obstructive Pulmonary Disease.2(2): 121–9.PMC2695611.PMID18044684.
- ^Herbert C, Hettiaratchi A, Webb DC, Thomas PS, Foster PS, Kumar RK (May 2008). "Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma".Clinical and Experimental Allergy.38(5): 847–56.doi:10.1111/j.1365-2222.2008.02950.x.PMID18307529.S2CID19050454.
- ^Field SK (May 2008). "Roflumilast: an oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma".Expert Opinion on Investigational Drugs.17(5): 811–8.doi:10.1517/13543784.17.5.811.PMID18447606.S2CID73241684.
- ^"2022 First Generic Drug Approvals".U.S.Food and Drug Administration(FDA).3 March 2023.Archivedfrom the original on 30 June 2023.Retrieved30 June2023.
- ^"FDA Approves Arcutis' Zoryve (Roflumilast) Cream 0.3% For the Treatment of Plaque Psoriasis in Individuals Age 12 and Older"(Press release). Arcutis Biotherapeutics. 29 July 2022.Archivedfrom the original on 1 August 2022.Retrieved1 August2022– via GlobeNewswire.
- ^"FDA Approves Atopic Dermatitis Label Expansion for Arcutis' Zoryve Cream".BioSpace.Retrieved13 July2024.
- ^Spina D (October 2008)."PDE4 inhibitors: current status".British Journal of Pharmacology.155(3): 308–15.doi:10.1038/bjp.2008.307.PMC2567892.PMID18660825.
- ^""Nycomed's Anti-Inflammatory Gains Approval in EU for COPD"".Archivedfrom the original on 24 August 2017.Retrieved10 July2010.
- ^"Drug Approval Package: Daliresp Tablets (roflumilast) NDA #022522".U.S.Food and Drug Administration(FDA).24 December 1999.Archivedfrom the original on 27 October 2020.Retrieved28 September2020.
- ^"FDA approves new drug to treat chronic obstructive pulmonary disease"(Press release). U.S.Food and Drug Administration(FDA). 1 March 2011. Archived fromthe originalon 18 January 2017.Retrieved16 December2019.
Further reading
[edit]- Hohlfeld JM, Schoenfeld K, Lavae-Mokhtari M, Schaumann F, Mueller M, Bredenbroeker D, et al. (August 2008)."Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: a randomized placebo-controlled trial"(PDF).Pulmonary Pharmacology & Therapeutics.21(4): 616–23.doi:10.1016/j.pupt.2008.02.002.PMID18374614.Archived(PDF)from the original on 28 August 2019.Retrieved24 June2019.
