Rumenic acid
| |
| Names | |
|---|---|
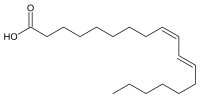
| Preferred IUPAC name
(9Z,11E)-Octadeca-9,11-dienoic acid | |
| Other names
Bovinic acid; C9-T11 acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C18H32O2 | |
| Molar mass | 280.452g·mol−1 |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Rumenic acid,also known asbovinic acid,is aconjugated linoleic acid(CLA) found in the fat ofruminantsand indairy products.It is anOmega -7trans fatty acid.Its lipid shorthand name is cis-9, trans-11 18:2 acid. The name was proposed by Krameret al.in 1998.[1]It can be considered as the principal dietary form, accounting for as much as 85-90% of the totalCLAcontent in dairy products.[2]
Biosynthesis and biotransformations[edit]
Rumenic acid is produced fromvaccenic acidby the action of unsaturase enzymes.[3]Rumenic acid is converted back to vaccenic acid en route tostearic acid
Further reading[edit]
F. Destaillats; E. Buyukpamukcu; P.-A. Golay; F. Dionisi & F. Giuffrida (2005)."Letter to the Editor: Vaccenic and Rumenic Acids, A Distinct Feature of Ruminant Fats".Journal of Dairy Science.88(449): 449.doi:10.3168/jds.S0022-0302(05)72705-3.PMID15653508.
References[edit]
- ^Kramer J, Parodi P, Jensen R, Mossoba M, Yurawecz M, Adlof R (1998). "Rumenic acid: a proposed common name for the major conjugated linoleic acid isomer found in natural products".Lipids.33(8): 835.doi:10.1007/s11745-998-0279-6.PMID9727617.S2CID10693714.
- ^Cyberlipid."Polyenoic Fatty Acids".Retrieved2007-01-17.
- ^Turpeinen, Anu M.; Mutanen, Marja; Aro, Antti; Salminen, Irma; Basu, Samar; Palmquist, Donald L.; Griinari, J Mikko (2002)."Bioconversion of vaccenic acid to conjugated linoleic acid in humans".The American Journal of Clinical Nutrition.76(3): 504–510.doi:10.1093/ajcn/76.3.504.PMID12197992.
