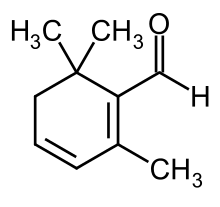
Safranal
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,6,6-Trimethylcyclohexa-1,3-diene-1-carbaldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.758 |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C10H14O | |
| Molar mass | 150.21 g/mol |
| Density | 0.9734 g/cm3 |
| Boiling point | 70 °C (158 °F; 343 K) at 1 mmHg |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Safranalis anorganic compoundisolated fromsaffron,the spice consisting of thestigmasofcrocusflowers (Crocus sativus). It is the constituent primarily responsible for the aroma of saffron.
It is believed that safranal is a degradation product of thecarotenoidzeaxanthinvia the intermediatepicrocrocin.
Pharmacology[edit]
Safranal is an effective anticonvulsant in animal models, shown to act as anagonistatGABAAreceptors.[1][2]Safranal also exhibits highantioxidantand free radical scavenging activity,[3][4]along withcytotoxicitytowards cancer cellsin vitro.[5]One of its anticancer mechanisms of action involves disruption of the normal assembly dynamics of cellular microtubules.[6]It has also been shown to have antidepressant properties in animals and pilot studies in humans.[7][8]
Natural sources[edit]
Natural sources of safranal include:[9][unreliable source?]
- Microcystis(cyanobacterium)
- Aspalathus linearis(Rooibos)
- Camellia sinensis(Tea leaf)
- Crocus sativus(Saffron)
- Ficus carica(Fig leaf)
- Lycium chinense(Wolfberry)
- Cuminum cyminum(Cumin Seed)[10]
- Centaurea sibthorpii[11]
- Centaurea amanicola[11]
- Centaurea consanguinea[11]
- Erodium cicutarium(common stork's-bill or pinweed)[11]
- Calycopteris floribunda(Ukshi)[11]
- Sambucus nigra(elderberry)[11]
- Citrus limon(lemon)[11]
- Achillea distans[11]
References[edit]
- ^Hosseinzadeh H; Talebzadeh F (December 2005). "Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice".Fitoterapia.76(7–8): 722–4.doi:10.1016/j.fitote.2005.07.008.PMID16253437.
- ^Hosseinzadeh H; Sadeghnia HR (April 2007). "Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: involvement of GABAergic and opioids systems".Phytomedicine.14(4): 256–62.doi:10.1016/j.phymed.2006.03.007.PMID16707256.
- ^Hosseinzadeh H; Sadeghnia HR (2005)."Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus".Journal of Pharmacy & Pharmaceutical Sciences.8(3): 394–9.PMID16401389.
- ^Assimopoulou AN; Sinakos Z; Papageorgiou VP (November 2005). "Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents".Phytotherapy Research.19(11): 997–1000.doi:10.1002/ptr.1749.PMID16317646.S2CID23907085.
- ^Escribano J; Alonso GL; Coca-Prados M; Fernandez JA (February 1996)."Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro".Cancer Letters.100(1–2): 23–30.doi:10.1016/0304-3835(95)04067-6.PMID8620447.
- ^Cheriyamundath S, Choudhary S, and Lopus M (2017) Safranal inhibits HeLa cell viability by perturbing the reassembly potential of microtubules. Phytother Res, 32, 170-173. doi: 10.1002/ptr.5938.PMID29024138
- ^Hosseinzadeh H; Karimi G; Niapoor M (2004)."Antidepressant effect ofCrocus sativusL. stigma extracts and their constituents, crocin and safranal, in mice ".Acta Horticulturae.650(650): 435–45.doi:10.17660/ActaHortic.2004.650.54.
- ^Akhondzadeh S; Fallah-Pour H; Afkham K; Jamshidi AH; Khalighi-Cigaroudi F (September 2004)."Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial ISRCTN45683816".BMC Complementary and Alternative Medicine.4:12.doi:10.1186/1472-6882-4-12.PMC517724.PMID15341662.
- ^ "List of Chemicals".sun.ars-grin.gov.Retrieved2008-03-02.
- ^Yan JH; Tang KW; Zhong M; Deng NH (November 2002). "[Determination of chemical components of volatile oil from Cuminum cyminum L. by gas chromatography-mass spectrometry]".Se Pu(in Chinese).20(6): 569–72.PMID12683011.
- ^abcdefghRamin Rezaee; Hossein Hosseinzadeh (January 2013)."[Safranal: From an Aromatic Natural Product to a Rewarding Pharmacological Agent]".Iranian Journal of Basic Medical Sciences.16(1): 12–26.PMC3637901.PMID23638289.
