Silenes
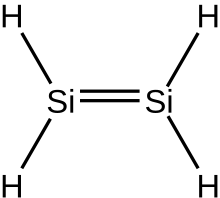
Ininorganic chemistry,silenes,ordisilalkenes,[1]aresiliconcompounds that containSi=Sidouble bonds.Theparent moleculeisdisilene,Si2H4.
Structure
[edit]The first transient disilene was reported in 1972 by D. N. Roark and Garry J. D. Peddle. Simple disilenes easilypolymerize.To suppress this tendency, bulkysubstituentsare used. Indeed the first isolable disilene, tetramesityldisilene, was described in 1981 byWest,Fink, and Michl.[2][3]It was prepared by UV-photolysisof the relatedcyclictrisilane:
- 2 [Si(mesityl)2]3→ 3 (mesityl)2Si=Si(mesityl)2
Structure of tetramesityldisilene
[edit]Tetramesityldisilene(CH3)2Si=Si(CH3)2is a yellow-orange solid. The Si=Sidouble bondlengths of disilenes vary between 2.14 and 2.29 Å and are nearly 5 to 10% shorter than the Si-Sisingle bondlengths of corresponding disilanes. A peculiarity of disilenes is thetrans-bending of thesubstituents,which is never observed inalkenes.Thetrans-bent angles of disilenes between the R2Si planes and the Si=Si vector range from 0 to 33.8 °. This distortion is rationalized by the stability of the correspondingsilylenefragments, although disilenes do not typically dissociate.
The distorted geometry of disilenes can be rationalized by considering thevalenceorbitalsofsilicon,which are3sand 3p, whereas those ofcarbonare 2s and 2p. Thus, the energy gap between thensandnp orbitalsof asiliconatomis larger than that of acarbonatom. Therefore,silylenefragments are in asingletstate, whilecarbenefragments are in atripletstate. So, when double bonds are formed by the interaction of these two fragments, disilenes which consist of twosilyleneunits aretrans-bending andalkeneswhich consist of twocarbeneunits are planar. The bending is even more extreme for the tin analogues of disilenes.[1]
Synthesis
[edit]Disilenes are generally synthesized byreductionof 1,2-dihalodisilane, byretro-Diels–Alderfragmentation, bydimerizationofsilylenes,by photofragmentation ofcyclopolysilanes,or by rearrangement of silylsilylenes.
A series of 1,1,1,4,4,4-hexaalkyl-2,3-bis(trialkylsilyl)tetrasil-2-enes structural analogues are typically synthesised using reductive coupling of the corresponding 1,1,1,3,3,3-hexaalkyl-2,3-tribromotrisilanes.
IUPAC names
[edit]To form the root of theIUPACnames for silenes, simply change the -an- infix of the parent to -en-. For example,SiH
3-SiH
3is the silanedisilANe.The name ofSiH
2=SiH
2is thereforedisilENe.
In higher silenes, whereisomersexist that differ in location of the double bond, the following numbering system is used:
- Number the longest silicon chain that contains the double bond in the direction that gives the silicon atoms of the double bond the lowest possible numbers.
- Indicate the location of the double bond by the location of its first silicon.
- Name branched or substituted silenes in a manner similar tosilanes.
- Number the silicon atoms, locate and name substituent groups, locate the double bond, and name the main chain.
See also
[edit]References
[edit]- ^abPhilip P. Power "pi-Bonding and the Lone Pair Effect in Multiple Bonds between Heavier Main Group Elements" Chemical Reviews, 1999, 99, 3462.doi:10.1021/cr9408989
- ^West, R.; Fink, M. J.; Michl, J.Tetramesityldisilene, a Stable Compound Containing a Silicon-Silicon Double BondScience 1981, Vol. 214, pp. 1343-1344.doi:10.1126/science.214.4527.1343.
- ^Greenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.p. 363.ISBN978-0-08-037941-8.
