Skeletal formula

Theskeletal formulaof anorganic compoundis a shorthand representation of itsmolecular structure,developed by the organic chemist,Friedrich August Kekulé von Stradonitz.Skeletal formulae are ubiquitous inorganic chemistry,because they are relatively quick and simple to draw. Carbon and hydrogen atoms are not shown explicitly. A skeletal formula shows theskeletal structureorskeletonof a molecule, which is composed ofskeletal atoms.
The skeleton
The skeletal structure of an organic compound is the string of connected atoms that form the essential structure of the compound. The skeleton can consist of chains, branches and/or rings. Skeletal atoms other than carbon are calledheteroatoms.[1]
The skeleton has hydrogen and/orsubstituentsbonded to its atoms. Hydrogen is the most common non-carbon atom bonded to carbon and is not explicitly drawn. Heteroatoms and definite groups of atoms are calledfunctional groups,as they give the molecule a function. Heteroatoms and functional groups are known collectively as substituents, as they are considered to be a substitute for the hydrogen atom that would be present in the parenthydrocarbonof the organic compound in question.
Implicit carbon and hydrogen atoms
For example, in the image below, the skeletal formula ofhexaneis shown. The carbon atom labelled C1has only one bond shown to it, so there must also be three hydrogens bonded to it, in order to make its total number of bonds four. The carbon atom labelled C3has two bonds to other carbons and is therefore bonded to two hydrogen atoms as well. Aball-and-stick modelof the actual molecular structure of hexane, as determined byX-ray crystallography,is shown for comparison, in which carbon atoms are depicted as black balls and hydrogen atoms as white ones.


Any hydrogen atoms bonded to non-carbon atomsaredrawn explicitly. Inethanol,C2H5OH, for instance, the hydrogen atom bonded to oxygen is denoted by the symbol H, whereas the hydrogen atoms which are bonded to carbon atoms are not shown directly. Lines representing heteroatom-hydrogen bonds are usually omitted for clarity and compactness, so a functional group like thehydroxylgroup is most often written −OH instead of −O−H. These bonds are sometimes drawn out in full in order to accentuate their presence when they participate inreaction mechanisms.
Shown below for comparison are a ball-and-stick model of the actual three-dimensional structure of the ethanol molecule in the gas phase (determined bymicrowave spectroscopy,left), theLewis structure(centre) and the skeletal formula (right).


Explicit heteroatoms
All atoms that are not carbon or hydrogen are signified by theirchemical symbol,for instance Cl forchlorine,O foroxygenand Na forsodium.These atoms are commonly known as heteroatoms in the context of organic chemistry.
Pseudoelement symbols
There are also symbols that appear to bechemical element symbols,but represent certain very common substituents or indicate an unspecified member of a group of elements. These are known as pseudoelement symbols or organic elements.[2]The most widely used symbol is Ph, which represents thephenyl group.A list of pseudoelement symbols is shown below:
Elements
Alkyl groups
- R for anyalkylgroup or even anysubstituentat all
- Me for themethyl group
- Et for theethyl group
- n-Pr for thepropylgroup
- i-Pr for theisopropylgroup
- Bu for thebutylgroup
- i-Bu for theisobutylgroup
- s-Bu for thesecondary butylgroup
- t-Bu for thetertiary butylgroup
- Pn for thepentylgroup
- Hx for thehexylgroup
- Hp for theheptylgroup
- Cy for thecyclohexylgroup
Aromatic substituents
- Ar for anyaromaticsubstituent
- Bn for thebenzylgroup
- Bz for thebenzoylgroup
- Mes for themesitylgroup
- Ph for thephenyl group
- Tol for thetolylgroup
- Cp for thecyclopentadienylgroup
- Cp* for thepentamethylcyclopentadienylgroup
Functional groups
- Ac for theacetylgroup(Ac is also the symbol for the elementactinium.However, actinium is almost never encountered in organic chemistry, so the use of Ac to represent the acetyl group never causes confusion)
Leaving groups
See the articleleaving groupfor further information
Multiple bonds
Two atoms can be bonded by sharing more than one pair of electrons. The common bonds to carbon are single, double and triple bonds. Single bonds are most common and are represented by a single, solid line between two atoms in a skeletal formula. Double bonds are denoted by two parallel lines, and triple bonds are shown by three parallel lines.
In more advanced theories of bonding, non-integervalues ofbond orderexist. In these cases, a combination of solid and dashed lines indicate the integer and non-integer parts of the bond order, respectively.
-
Hex-3-ene has an internalcarbon-carbon double bond -
Hex-1-enehas a terminal double bond -
Hex-3-ynehas an internalcarbon-carbon triple bond -
Hex-1-yne has a terminal carbon-carbon triple bond
N.B.in the gallery above, double bonds have been shown in red and triple bonds in blue. This was added for clarity - multiple bonds are not normally coloured in skeletal formulae.
Benzene rings

Benzenerings are common inorganic compounds.To represent thedelocalizationofelectronsover the six carbon atoms in the ring, a circle is drawn inside the hexagon of single bonds. This style is very common in introductory organic chemistry texts used in schools.

An alternative style that is more common in academia is theKekuléstructure. Although it could be considered inaccurate as it implies three single bonds and three double bonds (benzene would therefore be 1,3,5-cyclohexatriene), all qualifiedchemistsare fully aware of the delocalization in benzene. Kekulé structures are useful for drawingreaction mechanismsclearly.
Stereochemistry
Stereochemistryis conveniently denoted in skeletal formulae:[3]
- solid lines representbondsin the plane of the paper or screen
- wedges represent bonds that point out of the plane of the paper or screen, towards the observer
- dashed lines represent bonds that point into the plane of the paper or screen, away from the observer
- wavy lines represent either unknown stereochemistry or a mixture of the two possible stereoisomers at that point
-
2-chloro-2-fluoropentane -
The stereochemical skeletal formula of 2-chloro-2-fluoropentane -
The other possibility for 2-chloro-2-fluoropentane -
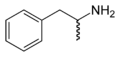
The skeletal formula ofamphetamine,a mixture of bothRandSstereoisomers
Skeletal formulae can depictcisandtransisomersof alkenes. Wavy single bonds are the standard way to represent unknown or unspecified stereochemistry or a mixture of isomers (as with tetrahedeal stereocenters). A crossed double-bond has been used sometimes, but is no longer considered an acceptable style.[3]
Hydrogen bonds
Hydrogen bondsare sometimes denoted by dotted or dashed lines
External links
- Drawing organic moleculesfromchemguide.co.uk
References
- ^IUPAC Recommendations 1999, Revised Section F:Replacement of Skeletal Atoms
- ^Clayden, Jonathan;Greeves, Nick;Warren, Stuart;Wothers, Peter(2001).Organic Chemistry(1st ed.). Oxford University Press. p. 27.ISBN978-0-19-850346-0.
- ^abJ. Brecher (2006)."Graphical representation of stereochemical configuration (IUPAC Recommendations 2006)".Pure Appl. Chem.78(10): 1897–1970.doi:10.1351/pac200678101897.