Solasulfone
Appearance
| |
| Names | |
|---|---|
| IUPAC name
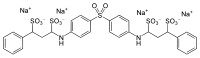
Tetrasodium 3,3'-[sulfonylbis(benzene-4,1-diylimino)]bis(1-phenylpropane-1,3-disulfonate)
| |
| Other names
Solapsone; Sulfetrone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.004.652 |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C30H28N2Na4O14S5 | |
| Molar mass | 892.81g·mol−1 |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Solasulfoneis anantileprotic drugdeveloped from the parent compound sulphetrone.[1]It was first described and evaluated in the 1930s and 1940s as an antibacterial agent for the treatment oftuberculosisand various other infections, and later found to be effective in the treatment ofleprosy.[2]
References
[edit]- ^Chemistry for Pharmacy and the Life Sciences(1996, Pearson Education Limited) Thomas, Gareth; ppg. 349-350
- ^Brownlee, George; Green, A. F.; Woodbine, M. (March 1948)."Sulphetrone*: A Chemotherapeutic Agent for Tuberculosis: Pharmacology and Chemotherapy".British Journal of Pharmacology and Chemotherapy.3(1): 15–28.doi:10.1111/j.1476-5381.1948.tb00348.x.PMC1509804.PMID18904720.
