Spectrometer
This articleneeds additional citations forverification.(November 2023) |

Aspectrometer(/spɛkˈtrɒmɪtər/) is a scientific instrument used to separate and measurespectralcomponents of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. Invisible lighta spectrometer can separate whitelightand measure individual narrow bands of color, called a spectrum. Amass spectrometermeasures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers weredevelopedin early studies ofphysics,astronomy,andchemistry.The capability ofspectroscopyto determinechemical compositiondrove its advancement and continues to be one of its primary uses. Spectrometers are used inastronomyto analyze the chemical composition ofstarsandplanets,and spectrometers gather data on theorigin of the universe.
Examples of spectrometers are devices that separateparticles,atoms,andmoleculesby theirmass,momentum,orenergy.These types of spectrometers are used inchemical analysisandparticle physics.
Types of spectrometer[edit]
Optical spectrometers or optical emission spectrometer[edit]
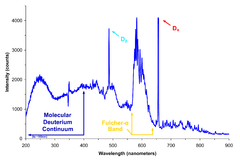
Optical absorption spectrometers[edit]
Optical spectrometers(often simply called "spectrometers" ), in particular, show the intensity oflightas a function of wavelength or of frequency. The different wavelengths of light are separated byrefractionin aprismor bydiffractionby adiffraction grating.Ultraviolet–visible spectroscopyis an example.
These spectrometers utilize the phenomenon ofoptical dispersion.The light from a source can consist of acontinuous spectrum,anemission spectrum(bright lines), or anabsorption spectrum(dark lines). Because each element leaves itsspectral signaturein the pattern of lines observed, aspectral analysiscan reveal the composition of the object being analyzed.[1]
A spectrometer that is calibrated for measurement of the incident optical power is called aspectroradiometer.[2]
Optical emission spectrometers[edit]
Optical emission spectrometers(often called "OES or spark discharge spectrometers" ), is used to evaluatemetalsto determine the chemical composition with very high accuracy. A spark is applied through a high voltage on the surface which vaporizes particles into a plasma. The particles and ions then emit radiation that is measured by detectors (photomultiplier tubes) at different characteristic wavelengths.
Electron spectroscopy[edit]
Some forms of spectroscopy involve analysis of electron energy rather than photon energy.X-ray photoelectron spectroscopyis an example.
Mass spectrometer[edit]
Amass spectrometeris an analytical instrument that is used to identify the amount and type of chemicals present in a sample by measuring themass-to-charge ratioand abundance of gas-phaseions.[3]
Time-of-flight spectrometer[edit]
The energy spectrum of particles of known mass can also be measured by determining the time of flight between twodetectors(and hence, the velocity) in atime-of-flight spectrometer.Alternatively, if the particle-energy is known, masses can be determined in atime-of-flight mass spectrometer.
Magnetic spectrometer[edit]

When a fastcharged particle(chargeq,massm) enters a constant magnetic fieldBat right angles, it is deflected into a circular path of radiusr,due to theLorentz force.The momentumpof the particle is then given by
- ,

wheremandvare mass and velocity of the particle. The focusing principle of the oldest and simplest magnetic spectrometer, the semicircular spectrometer,[4]invented by J. K. Danisz, is shown on the left. A constant magnetic field is perpendicular to the page. Charged particles of momentumpthat pass the slit are deflected into circular paths of radiusr = p/qB.It turns out that they all hit the horizontal line at nearly the same place, the focus; here a particle counter should be placed. VaryingB,this makes possible to measure the energy spectrum ofAlpha particlesin an Alpha particle spectrometer, ofbeta particlesin a beta particle spectrometer,[5]of particles (e.g., fastions) in a particle spectrometer, or to measure the relative content of the various masses in amass spectrometer.
Since Danysz' time, many types of magnetic spectrometers more complicated than the semicircular type have been devised.[5]
Resolution[edit]
Generally, theresolutionof an instrument tells us how well two close-lying energies (or wavelengths, or frequencies, or masses) can be resolved. Generally, for an instrument with mechanical slits, higher resolution will mean lower intensity.
See also[edit]
References[edit]
- ^
 OpenStax, Astronomy. OpenStax. 13 October 2016. <http://cnx.org/content/col11992/latest/>
OpenStax, Astronomy. OpenStax. 13 October 2016. <http://cnx.org/content/col11992/latest/>
- ^Schneider, T.; Young, R.; Bergen, T.; Dam-Hansen, C; Goodman, T.; Jordan, W.; Lee, D.-H; Okura, T.; Sperfeld, P.; Thorseth, A; Zong, Y. (2022).CIE 250:2022 Spectroradiometric Measurement of Optical Radiation Sources.Vienna: CIE - International Commission on Illumination.ISBN978-3-902842-23-7.
- ^"mass spectrometer"(PDF).IUPAC Compendium of Chemical Terminology.2009.doi:10.1351/goldbook.M03732.ISBN978-0-9678550-9-7.S2CID99611182.Archived fromthe original(PDF)on 2018-10-08.Retrieved2015-06-15.
- ^Jan Kazimierz Danysz,Le Radium 9, 1 (1912); 10, 4 (1913)
- ^abK. Siegbahn, Alpha-, Beta- and Gamma-Ray Spectroscopy, North-Holland Publishing Co. Amsterdam (1966)

