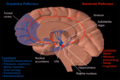
Substantia nigra
| Substantia nigra | |
|---|---|
 Substantia nigra highlighted in red. | |
 Section throughsuperior colliculusshowing substantia nigra. | |
| Details | |
| Part of | Midbrain,basal ganglia |
| Identifiers | |
| Latin | substantia nigra |
| Acronym(s) | SN |
| MeSH | D013378 |
| NeuroNames | 536 |
| NeuroLexID | birnlex_789 |
| TA98 | A14.1.06.111 |
| TA2 | 5881 |
| FMA | 67947 |
| Anatomical terms of neuroanatomy | |
Thesubstantia nigra(SN) is abasal gangliastructure located in themidbrainthat plays an important role inrewardandmovement.Substantia nigraisLatinfor "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels ofneuromelaninindopaminergicneurons.[1]Parkinson's diseaseis characterized by the loss of dopaminergic neurons in the substantia nigrapars compacta.[2]
Although the substantia nigra appears as a continuous band in brain sections, anatomical studies have found that it actually consists of two parts with very different connections and functions: thepars compacta(SNpc) and thepars reticulata(SNpr). The pars compacta serves mainly as a projection to the basal ganglia circuit, supplying thestriatumwith dopamine. The pars reticulata conveys signals from thebasal gangliato numerous other brain structures.[3]
Structure
[edit]

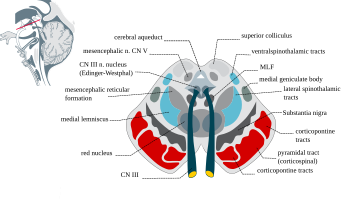
The substantia nigra, along with four other nuclei, is part of thebasal ganglia.It is the largestnucleusin the midbrain, lying dorsal to thecerebral peduncles.Humans have two substantiae nigrae, one on each side of the midline.
The SN is divided into two parts: thepars reticulata(SNpr) and thepars compacta(SNpc), which lies medial to the pars reticulata. Sometimes, a third region, the pars lateralis, is mentioned, though it is usually classified as part of the pars reticulata. The (SNpr) and the internalglobus pallidus(GPi) are separated by theinternal capsule.[4]
Pars reticulata
[edit]The pars reticulata bears a strong structural and functional resemblance to the internal part of the globus pallidus. The two are sometimes considered parts of the same structure, separated by the white matter of the internal capsule. Like those of the globus pallidus, the neurons in pars reticulata are mainlyGABAergic.[citation needed]
Afferent connections
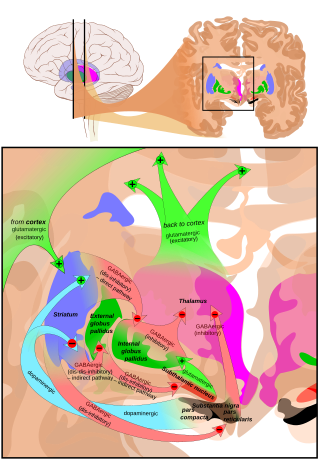
[edit]The main input to the SNpr derives from thestriatum.It comes by two routes, known as thedirectandindirect pathways.The direct pathway consists of axons from medium spiny cells in the striatum that project directly to pars reticulata. The indirect pathway consists of three links: a projection from striatal medium spiny cells to the external part of theglobus pallidus;aGABAergicprojection from the globus pallidus to thesubthalamic nucleus,and aglutamatergicprojection from the subthalamic nucleus to the pars reticulata.[5][better source needed]Thus, striatal activity via the direct pathway exerts an inhibitory effect on neurons in the (SNpr) but an excitatory effect via the indirect pathway. The direct and indirect pathways originate from different subsets of striatal medium spiny cells: They are tightly intermingled, but express different types of dopamine receptors, as well as showing other neurochemical differences.
Efferent connections
[edit]Significant projections occur to the thalamus (ventral lateral and ventral anterior nuclei),superior colliculus,and other caudal nuclei from the pars reticulata (the nigrothalamic pathway),[6]which use GABA as their neurotransmitter. In addition, these neurons form up to five collaterals that branch within both the pars compacta and pars reticulata, likely modulating dopaminergic activity in the pars compacta.[7]
Function
[edit]The substantia nigra is an important player in brain function, in particular, ineye movement,motor planning,reward-seeking,learning,andaddiction.Many of the substantia nigra's effects are mediated through thestriatum.The nigraldopaminergicinput to the striatum via thenigrostriatal pathwayis intimately linked with the striatum's function.[8]The co-dependence between the striatum and substantia nigra can be seen in this way: when the substantia nigra is electrically stimulated, no movement occurs; however, the symptoms of nigral degeneration due to Parkinson's is a poignant example of the substantia nigra's influence on movement. In addition to striatum-mediated functions, the substantia nigra also serves as a major source ofGABAergicinhibition to various brain targets.
Pars reticulata
[edit]Thepars reticulataof the substantia nigra is an important processing center in the basal ganglia. The GABAergic neurons in the pars reticulata convey the final processed signals of thebasal gangliato thethalamusandsuperior colliculus.In addition, the pars reticulata also inhibits dopaminergic activity in thepars compactavia axon collaterals, although the functional organization of these connections remains unclear.
The GABAergic neurons of the pars reticulata spontaneously fireaction potentials.In rats, the frequency of action potentials is roughly 25 Hz.[9]The purpose of these spontaneous action potentials is to inhibit targets of the basal ganglia, and decreases in inhibition are associated with movement.[10]The subthalamic nucleus gives excitatory input that modulates the rate of firing of these spontaneous action potentials. However, lesion of the subthalamic nucleus leads to only a 20% decrease in pars reticulata firing rate, suggesting that the generation of action potentials in the pars reticulata is largely autonomous,[11]as exemplified by the pars reticulata's role insaccadic eye movement.A group of GABAergic neurons from the pars reticulata projects to the superior colliculus, exhibiting a high level of sustained inhibitory activity.[12]Projections from thecaudate nucleusto the superior colliculus also modulate saccadic eye movement. Altered patterns of pars reticulata firing such as single-spike or burst firing are found inParkinson's disease[13]andepilepsy.[14]
Pars compacta
[edit]The most prominent function of the pars compacta ismotor control,[15]though the substantia nigra's role in motor control is indirect; electrical stimulation of the substantia nigra does not result in movement, due to mediation of the striatum in the nigral influence of movement. The pars compacta sends excitatory input to the striatum via D1 pathway that excites and activates the striatum, resulting in the release of GABA onto the globus pallidus to inhibit its inhibitory effects on the thalamic nucleus. This causes the thalamocortical pathways to become excited and transmits motor neuron signals to the cerebral cortex to allow the initiation of movement, which is absent in Parkinson's disease. However, lack of pars compacta neurons has a large influence on movement, as evidenced by the symptoms of Parkinson's. The motor role of the pars compacta may involve fine motor control, as has been confirmed in animal models with lesions in that region.[16]
The pars compacta is heavily involved in learned responses to stimuli. In primates, dopaminergic neuron activity increases in the nigrostriatal pathway when a new stimulus is presented.[17]Dopaminergic activity decreases with repeated stimulus presentation.[17]However, behaviorally significant stimulus presentation (i.e. rewards) continues to activate dopaminergic neurons in the substantia nigra pars compacta. Dopaminergic projections from theventral tegmental area(bottom part of the "midbrain" or mesencephalon) to the prefrontal cortex (mesocortical pathway) and to the nucleus accumbens (mesolimbic pathway – "meso" referring to "from the mesencephalon"... specifically theventral tegmental area) are implicated in reward, pleasure, and addictive behavior. The pars compacta is also important in spatial learning, the observations about one's environment and location in space. Lesions in the pars compacta lead to learning deficits in repeating identical movements,[18]and some studies point to its involvement in a dorsal striatal-dependent, response-based memory system that functions relatively independent of thehippocampus,which is traditionally believed to subserve spatial orepisodic-like memoryfunctions.[19]
The pars compacta also plays a role intemporal processingand is activated during time reproduction.Lesionsin the pars compacta leads to temporal deficits.[20]As of late, the pars compacta has been suspected of regulating the sleep-wake cycle,[21]which is consistent with symptoms such asinsomniaandREM sleepdisturbances that are reported by patients withParkinson's disease.Even so, partial dopamine deficits that do not affect motor control can lead to disturbances in the sleep-wake cycle, especially REM-like patterns of neural activity while awake, especially in thehippocampus.[22]
Clinical significance
[edit]The substantia nigra is critical in the development of many diseases and syndromes, includingparkinsonismandParkinson's disease.There exist a study showing that high-frequency stimulation delivery to the left substantia nigra can induce transient acute depression symptoms.[23]
Parkinson's disease
[edit]
Parkinson's disease is aneurodegenerative diseasecharacterized, in part, by the death of dopaminergic neurons in the SNpc. The major symptoms of Parkinson's disease includetremor,akinesia,bradykinesia,and stiffness.[24]Other symptoms include disturbances to posture,fatigue,sleep abnormalities,anddepressed mood.[25]
The cause of death of dopaminergic neurons in the SNpc is unknown. However, some contributions to the unique susceptibility of dopaminergic neurons in the pars compacta have been identified. For one, dopaminergic neurons show abnormalities inmitochondrial complex 1,causing aggregation ofAlpha -synuclein;this can result in abnormal protein handling and neuron death.[26]Secondly, dopaminergic neurons in the pars compacta contain lesscalbindinthan other dopaminergic neurons.[27]Calbindinis a protein involved incalciumion transport within cells, and excess calcium in cells is toxic. Thecalbindintheory would explain the high cytotoxicity of Parkinson's in the substantia nigra compared to the ventral tegmental area. Regardless of the cause of neuronal death, the plasticity of the pars compacta is very robust; Parkinsonian symptoms do not generally appear until at least 30% of pars compacta dopaminergic neurons have died.[28]Most of this plasticity occurs at the neurochemical level; dopamine transport systems are slowed, allowing dopamine to linger for longer periods of time in the chemical synapses in the striatum.[29]
Menke, Jbabdi, Miller, Matthews and Zari (2010) used diffusion tensor imaging, as well as T1 mapping to assess volumetric differences in the SNpc and SNpr, in participants with Parkinson's compared to healthy individuals. These researchers found that participants with Parkinson's consistently had a smaller substantia nigra, specifically in the SNpr. Because the SNpr is connected to the posterior thalamus, ventral thalamus and specifically, the motor cortex, and because participants with Parkinson's disease report having a smaller SNprs (Menke, Jbabdi, Miller, Matthews and Zari, 2010), the small volume of this region may be responsible for motor impairments found in Parkinson's disease patients. This small volume may be responsible for weaker and/or less controlled motor movements, which may result in the tremors often experienced by those with Parkinson's.[30]
Oxidative stressand oxidative damage in the SNpc are likely key drivers in the etiology ofParkinson's diseaseas individuals age.[31]DNA damagescaused by oxidative stress can berepairedby processes modulated byAlpha -synuclein.[32]Alpha synuclein is expressed in the substantia nigra, but itsDNA repairfunction appears to be compromised inLewy bodyinclusion bearingneurons.[32]This loss may trigger cell death.
Schizophrenia
[edit]Increased levels of dopamine have long been implicated in the development ofschizophrenia.[33]However, much debate continues to this day surrounding thisdopamine hypothesis of schizophrenia.Despite the controversy, dopamine antagonists remain a standard and successful treatment for schizophrenia. These antagonists includefirst generation (typical) antipsychoticssuch asbutyrophenones,phenothiazines,andthioxanthenes.These drugs have largely been replaced bysecond-generation (atypical) antipsychoticssuch asclozapineandpaliperidone.In general, these drugs do not act on dopamine-producing neurons themselves, but on the receptors on the post-synaptic neuron.
Other, non-pharmacological evidence in support of the dopamine hypothesis relating to the substantia nigra include structural changes in the pars compacta, such as reduction in synaptic terminal size.[34]Other changes in the substantia nigra include increased expression ofNMDA receptorsin the substantia nigra, and reduceddysbindinexpression. Increased NMDA receptors may point to the involvement ofglutamate-dopamineinteractions in schizophrenia. Dysbindin, which has been (controversially) linked to schizophrenia, may regulate dopamine release, and low expression of dysbindin in the substantia nigra may be important in schizophrenia etiology.[35]Due to the changes to the substantia nigra in the schizophrenic brain, it may eventually be possible to use specific imaging techniques (such as neuromelanin-specific imaging) to detect physiological signs of schizophrenia in the substantia nigra.[36]
Wooden Chest Syndrome
[edit]Wooden chest,also called fentanyl chest wall rigidity syndrome, is a rare side effect of syntheticopioidssuch asFentanyl,Sulfentanil,Alfentanil,Remifentanil.It results in a generalised increase in skeletalmuscle tone.The mechanism is thought to be via increased dopamine release and decreased GABA release in the nerves of the substantia nigra/striatum. The effect is most pronounced on the chest wall muscles and can lead to impaired ventilation. The condition is most commonly observed in anaesthesia where rapid and high doses of these drugs are given intravenously.[citation needed]
Multiple system atrophy
[edit]Multiple system atrophycharacterized by neuronal degeneration in the striatum and substantia nigra was previously calledstriatonigral degeneration.
Chemical modification of the substantia nigra
[edit]Chemical manipulation and modification of the substantia nigra is important in the fields ofneuropharmacologyandtoxicology.Various compounds such as levodopa and MPTP are used in the treatment and study of Parkinson's disease, and many other drugs have effects on the substantia nigra.
Amphetamine and trace amines
[edit]Studies have shown that, in certain brain regions, amphetamine and trace amines increase the concentrations of dopamine in thesynaptic cleft,thereby heightening the response of the post-synaptic neuron.[37]The various mechanisms by which amphetamine and trace amines affect dopamine concentrations have been studied extensively, and are known to involve bothDATandVMAT2.[37][38][39]Amphetamine is similar in structure to dopamine and trace amines; as a consequence, it can enter the presynaptic neuron viaDATas well as by diffusing through the neural membrane directly.[37]Upon entering the presynaptic neuron, amphetamine and trace amines activateTAAR1,which, throughprotein kinasesignaling, induces dopamine efflux,phosphorylation-dependentDATinternalization,and non-competitive reuptake inhibition.[37][40]Because of the similarity between amphetamine and trace amines, it is also a substrate for monoamine transporters; as a consequence, it (competitively) inhibits the reuptake of dopamine and other monoamines by competing with them for uptake, as well.[37]
In addition, amphetamine and trace amines are substrates for the neuronal vesicular monoamine transporter,vesicular monoamine transporter 2(VMAT2).[39]When amphetamine is taken up byVMAT2,the vesicle releases (effluxes) dopamine molecules into the cytosol in exchange.[39]
Cocaine
[edit]Cocaine's mechanism of action in the human brain includes the inhibition of dopamine reuptake,[41]which accounts for cocaine's addictive properties, as dopamine is the critical neurotransmitter for reward. However, cocaine is more active in the dopaminergic neurons of theventral tegmental areathan the substantia nigra. Cocaine administration increases metabolism in the substantia nigra, which can explain the altered motor function seen in cocaine-using subjects.[42]The inhibition of dopamine reuptake by cocaine also inhibits the firing of spontaneous action potentials by the pars compacta.[43]The mechanism by which cocaine inhibits dopamine reuptake involves its binding to thedopamine transporterprotein. However, studies show that cocaine can also cause a decrease in DATmRNAlevels,[44]most likely due to cocaine blocking dopamine receptors rather than direct interference with transcriptional or translational pathways.[44]
Inactivation of the substantia nigra could prove to be a possible treatment for cocaine addiction. In a study of cocaine-dependent rats, inactivation of the substantia nigra via implantedcannulaegreatly reduced cocaine addiction relapse.[45]
Levodopa
[edit]The substantia nigra is the target of chemical therapeutics for the treatment of Parkinson's disease.Levodopa(commonly referred to as L-DOPA), the dopamine precursor, is the most commonly prescribed medication for Parkinson's disease, despite controversy concerning theneurotoxicityof dopamine and L-DOPA.[46]The drug is especially effective in treating patients in the early stages of Parkinson's, although it does lose its efficacy over time.[47]Levodopa can cross theblood–brain barrierand increases dopamine levels in the substantia nigra, thus alleviating the symptoms of Parkinson's disease. The drawback of levodopa treatment is that it treats the symptoms of Parkinson's (low dopamine levels), rather than the cause (the death of dopaminergic neurons in the substantia nigra).
MPTP
[edit]MPTP,is aneurotoxinspecific to dopaminergic cells in the brain, specifically in the substantia nigra. MPTP was brought to the spotlight in 1982 when heroin users in California displayed Parkinson's-like symptoms after usingMPPPcontaminated with MPTP. The patients, who were rigid and almost completely immobile, responded to levodopa treatment. No remission of the Parkinson's-like symptoms was reported, suggesting irreversible death of the dopaminergic neurons.[48]The proposed mechanism of MPTP involves disruption ofmitochondrialfunction, including disruption ofmetabolismand creation offree radicals.[49]
Soon after, MPTP was tested in animal models for its efficacy in inducing Parkinson's disease (with success). MPTP induced akinesia, rigidity, and tremor in primates, and its neurotoxicity was found to be very specific to the substantia nigra pars compacta.[50]In other animals, such as rodents, the induction of Parkinson's by MPTP is incomplete or requires much higher and frequent doses than in primates. Today, MPTP remains the most favored method to induce Parkinson's disease inanimal models.[49][51]
History
[edit]The substantia nigra was discovered in 1784 byFélix Vicq-d'Azyr,[52]andSamuel Thomas von Sömmerringalluded to this structure in 1791.[53]The differentiation between the substantia nigra pars reticulata and compacta was first proposed by Sano in 1910.[54]In 1963,Oleh Hornykiewiczconcluded from his observation that "cell loss in the substantia nigra (of Parkinson's disease patients) could well be the cause of the dopamine deficit in the striatum."[55]
Additional images
[edit]-
Dopamine and serotonin
-
Degradation of substantia nigra associated with Parkinson's disease.
-
Horizontal MRI (T1 weighted) slice with highlighting indicating location of the substantia nigra.
-
Enhanced Neuromelanin MRI with Color images (RGB) showing Substantia nigra pars compacta
-
Microfilming
References
[edit]- ^Rabey JM, Hefti F (1990). "Neuromelanin synthesis in rat and human substantia nigra".Journal of Neural Transmission. Parkinson's Disease and Dementia Section.2(1): 1–14.doi:10.1007/BF02251241.PMID2357268.S2CID6769760.
- ^Kim SJ, Sung JY, Um JW, Hattori N, Mizuno Y, Tanaka K, Paik SR, Kim J, Chung KC (October 2003)."Parkin cleaves intracellular Alpha -synuclein inclusions via the activation of calpain".The Journal of Biological Chemistry.278(43): 41890–9.doi:10.1074/jbc.M306017200.PMID12917442.
- ^Bolam, J. P.; Brown, M. T. C.; Moss, J.; Magill, P. J. (1 January 2009),"Basal Ganglia: Internal Organization",in Squire, Larry R. (ed.),Encyclopedia of Neuroscience,Oxford: Academic Press, pp. 97–104,doi:10.1016/b978-008045046-9.01294-8,ISBN978-0-08-045046-9,retrieved7 September2020
- ^Kita H, Jaeger D (2016). "Organization of the Globus Pallidus".Handbook of Basal Ganglia Structure and Function, Second Edition.Handbook of Behavioral Neuroscience. Vol. 24. pp. 259–276.doi:10.1016/B978-0-12-802206-1.00013-1.ISBN9780128022061.
- ^Nauta HJ, Cole M (July 1978). "Efferent projections of the subthalamic nucleus: an autoradiographic study in monkey and cat".The Journal of Comparative Neurology.180(1): 1–16.doi:10.1002/cne.901800102.PMID418083.S2CID43046462.
- ^Carpenter MB, Nakano K, Kim R (February 1976). "Nigrothalamic projections in the monkey demonstrated by autoradiographic technics".The Journal of Comparative Neurology.165(4): 401–15.doi:10.1002/cne.901650402.PMID57125.S2CID11790266.
- ^Deniau JM, Kitai ST, Donoghue JP, Grofova I (1982). "Neuronal interactions in the substantia nigra pars reticulata through axon collaterals of the projection neurons. An electrophysiological and morphological study".Experimental Brain Research.47(1): 105–13.doi:10.1007/BF00235891.PMID6288427.S2CID20289802.
- ^Nicola SM,Surmeier J,Malenka RC (2000). "Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens".Annual Review of Neuroscience.23:185–215.doi:10.1146/annurev.neuro.23.1.185.PMID10845063.
- ^Gernert M, Fedrowitz M, Wlaz P, Löscher W (November 2004)."Subregional changes in discharge rate, pattern, and drug sensitivity of putative GABAergic nigral neurons in the kindling model of epilepsy".The European Journal of Neuroscience.20(9): 2377–86.doi:10.1111/j.1460-9568.2004.03699.x.PMID15525279.S2CID24485657.
- ^Sato M, Hikosaka O (March 2002)."Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement".The Journal of Neuroscience.22(6): 2363–73.doi:10.1523/JNEUROSCI.22-06-02363.2002.PMC6758246.PMID11896175.
- ^Zahr NM, Martin LP, Waszczak BL (November 2004). "Subthalamic nucleus lesions alter basal and dopamine agonist stimulated electrophysiological output from the rat basal ganglia".Synapse.54(2): 119–28.doi:10.1002/syn.20064.PMID15352137.S2CID10239473.
- ^Hikosaka O, Wurtz RH (May 1983). "Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses".Journal of Neurophysiology.49(5): 1268–84.doi:10.1152/jn.1983.49.5.1268.PMID6864250.
- ^Tseng KY, Riquelme LA, Belforte JE, Pazo JH, Murer MG (January 2000). "Substantia nigra pars reticulata units in 6-hydroxydopamine-lesioned rats: responses to striatal D2 dopamine receptor stimulation and subthalamic lesions".The European Journal of Neuroscience.12(1): 247–56.doi:10.1046/j.1460-9568.2000.00910.x.hdl:11336/39220.PMID10651879.S2CID22886675.
- ^Deransart C, Hellwig B, Heupel-Reuter M, Léger JF, Heck D, Lücking CH (December 2003). "Single-unit analysis of substantia nigra pars reticulata neurons in freely behaving rats with genetic absence epilepsy".Epilepsia.44(12): 1513–20.doi:10.1111/j.0013-9580.2003.26603.x.PMID14636321.S2CID6661257.
- ^Hodge GK, Butcher LL (August 1980). "Pars compacta of the substantia nigra modulates motor activity but is not involved importantly in regulating food and water intake".Naunyn-Schmiedeberg's Archives of Pharmacology.313(1): 51–67.doi:10.1007/BF00505805.PMID7207636.S2CID24642979.
- ^Pioli EY, Meissner W, Sohr R, Gross CE, Bezard E, Bioulac BH (June 2008). "Differential behavioral effects of partial bilateral lesions of ventral tegmental area or substantia nigra pars compacta in rats".Neuroscience.153(4): 1213–24.doi:10.1016/j.neuroscience.2008.01.084.PMID18455318.S2CID11239586.
- ^abLjungberg T, Apicella P, Schultz W (January 1992). "Responses of monkey dopamine neurons during learning of behavioral reactions".Journal of Neurophysiology.67(1): 145–63.doi:10.1152/jn.1992.67.1.145.PMID1552316.S2CID18024404.
- ^Da Cunha C, Silva MH, Wietzikoski S, Wietzikoski EC, Ferro MM, Kouzmine I, Canteras NS (December 2006). "Place learning strategy of substantia nigra pars compacta-lesioned rats".Behavioral Neuroscience.120(6): 1279–84.doi:10.1037/0735-7044.120.6.1279.PMID17201473.
- ^Da Cunha C, Wietzikoski S, Wietzikoski EC, Miyoshi E, Ferro MM, Anselmo-Franci JA, Canteras NS (May 2003). "Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system".Neurobiology of Learning and Memory.79(3): 236–42.doi:10.1016/S1074-7427(03)00008-X.PMID12676522.S2CID12045200.
- ^Matell MS, Meck WH (January 2000). "Neuropsychological mechanisms of interval timing behavior".BioEssays.22(1): 94–103.doi:10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E.PMID10649295.
- ^Lima MM, Andersen ML, Reksidler AB, Vital MA, Tufik S (June 2007). Brosnan S (ed.)."The role of the substantia nigra pars compacta in regulating sleep patterns in rats".PLOS ONE.2(6): e513.Bibcode:2007PLoSO...2..513L.doi:10.1371/journal.pone.0000513.PMC1876809.PMID17551593.

- ^Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA (October 2006)."Dopaminergic control of sleep-wake states".The Journal of Neuroscience.26(41): 10577–89.doi:10.1523/JNEUROSCI.1767-06.2006.PMC6674686.PMID17035544.
- ^Bejjani, Boulos-Paul; Damier, Philippe; Arnulf, Isabelle; Thivard, Lionel; Bonnet, Anne-Marie; Dormont, Didier; Cornu, Philippe; Pidoux, Bernard; Samson, Yves; Agid, Yves (13 May 1999)."Transient Acute Depression Induced by High-Frequency Deep-Brain Stimulation".New England Journal of Medicine.340(19): 1476–1480.doi:10.1056/NEJM199905133401905.ISSN0028-4793.PMID10320386.
- ^Jankovic J (April 2008)."Parkinson's disease: clinical features and diagnosis".Journal of Neurology, Neurosurgery, and Psychiatry.79(4): 368–76.doi:10.1136/jnnp.2007.131045.PMID18344392.
- ^Adler CH (2005). "Nonmotor complications in Parkinson's disease".Movement Disorders.20(Suppl 11): S23-9.doi:10.1002/mds.20460.PMID15822106.S2CID19045599.
- ^Dawson TM,Dawson VL (October 2003). "Molecular pathways of neurodegeneration in Parkinson's disease".Science.302(5646): 819–22.Bibcode:2003Sci...302..819D.doi:10.1126/science.1087753.PMID14593166.S2CID35486083.
- ^Liang CL, Sinton CM, Sonsalla PK, German DC (December 1996). "Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration".Neurodegeneration.5(4): 313–8.doi:10.1006/neur.1996.0042.PMID9117542.
- ^Grosch J, Winkler J, Kohl Z (2016)."Early Degeneration of Both Dopaminergic and Serotonergic Axons – A Common Mechanism in Parkinson's Disease".Front. Cell. Neurosci.10:293.doi:10.3389/fncel.2016.00293.PMC5177648.PMID28066188.
- ^Interview. Yoland Smith, PhD[verification needed]
- ^Menke RA, Jbabdi S, Miller KL, Matthews PM, Zarei M (October 2010). "Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson's disease".NeuroImage.52(4): 1175–80.doi:10.1016/j.neuroimage.2010.05.086.PMID20677376.S2CID19871414.
- ^Trist BG, Hare DJ, Double KL. Oxidative stress in the aging substantia nigra and the etiology of Parkinson's disease. Aging Cell. 2019 Dec;18(6):e13031. doi: 10.1111/acel.13031. Epub 2019 Aug 20. Review.PMID31432604
- ^abSchaser AJ, Osterberg VR, Dent SE, Stackhouse TL, Wakeham CM, Boutros SW, Weston LJ, Owen N, Weissman TA, Luna E, Raber J, Luk KC, McCullough AK, Woltjer RL, Unni VK. Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders. Sci Rep. 2019 Jul 29;9(1):10919. doi: 10.1038/s41598-019-47227-z.PMID31358782
- ^van Rossum J (1967). "The significance of dopamine-receptor blockade for the action of neuroleptic drugs". In Brill H, Cole J, Deniker P, Hippius H, Bradley PB (eds.).Neuropsychopharmacology, Proceedings Fifth Collegium Internationale Neuropsychopharmacologicum.pp. 321–9.OCLC458719.
- ^Kolomeets NS, Uranova NA (1999). "Synaptic contacts in schizophrenia: studies using immunocytochemical identification of dopaminergic neurons".Neuroscience and Behavioral Physiology.29(2): 217–21.doi:10.1007/BF02465329.PMID10432512.S2CID2233617.
- ^Kumamoto N, Matsuzaki S, Inoue K, Hattori T, Shimizu S, Hashimoto R, Yamatodani A, Katayama T, Tohyama M (June 2006). "Hyperactivation of midbrain dopaminergic system in schizophrenia could be attributed to the down-regulation of dysbindin".Biochemical and Biophysical Research Communications.345(2): 904–9.doi:10.1016/j.bbrc.2006.04.163.PMID16701550.
- ^Shibata E, Sasaki M, Tohyama K, Otsuka K, Endoh J, Terayama Y, Sakai A (September 2008). "Use of neuromelanin-sensitive MRI to distinguish schizophrenic and depressive patients and healthy individuals based on signal alterations in the substantia nigra and locus ceruleus".Biological Psychiatry.64(5): 401–6.doi:10.1016/j.biopsych.2008.03.021.PMID18452894.S2CID25752976.
- ^abcdeMiller GM (January 2011)."The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity".Journal of Neurochemistry.116(2): 164–76.doi:10.1111/j.1471-4159.2010.07109.x.PMC3005101.PMID21073468.
- ^"Amphetamine".DrugBank.University of Alberta. 8 February 2013.Retrieved13 October2013.
- ^abcEiden LE, Weihe E (January 2011)."VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse".Annals of the New York Academy of Sciences.1216(1): 86–98.Bibcode:2011NYASA1216...86E.doi:10.1111/j.1749-6632.2010.05906.x.PMC4183197.PMID21272013.
- ^Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP (March 2009)."International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature".Pharmacological Reviews.61(1): 1–8.doi:10.1124/pr.109.001107.PMC2830119.PMID19325074.
- ^Heikkila RE, Cabbat FS, Duvoisin RC (1979). "Motor activity and rotational behavior after analogs of cocaine: correlation with dopamine uptake blockade".Communications in Psychopharmacology.3(5): 285–90.PMID575770.
- ^Lakoski JM, Galloway MP, White FJ (1991).Cocaine.Telford Press.ISBN978-0-8493-8813-2.
- ^Lacey MG, Mercuri NB, North RA (April 1990)."Actions of cocaine on rat dopaminergic neurones in vitro".British Journal of Pharmacology.99(4): 731–5.doi:10.1111/j.1476-5381.1990.tb12998.x.PMC1917549.PMID2361170.
- ^abXia Y, Goebel DJ, Kapatos G, Bannon MJ (September 1992). "Quantitation of rat dopamine transporter mRNA: effects of cocaine treatment and withdrawal".Journal of Neurochemistry.59(3): 1179–82.doi:10.1111/j.1471-4159.1992.tb08365.x.PMID1494906.S2CID34068876.
- ^See RE, Elliott JC, Feltenstein MW (October 2007). "The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats".Psychopharmacology.194(3): 321–31.doi:10.1007/s00213-007-0850-8.PMID17589830.S2CID12652533.
- ^Cheng N, Maeda T, Kume T, Kaneko S, Kochiyama H, Akaike A, Goshima Y, Misu Y (December 1996). "Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons".Brain Research.743(1–2): 278–83.doi:10.1016/S0006-8993(96)01056-6.PMID9017256.S2CID22529926.
- ^Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL (2003). "Limitations of current Parkinson's disease therapy".Annals of Neurology.53(Suppl 3): S3–12, discussion S12–5.doi:10.1002/ana.10513.PMID12666094.S2CID45078589.
- ^Langston JW, Ballard P, Tetrud JW, Irwin I (February 1983). "Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis".Science.219(4587): 979–80.Bibcode:1983Sci...219..979L.doi:10.1126/science.6823561.PMID6823561.S2CID31966839.
- ^abSchmidt N, Ferger B (2001). "Neurochemical findings in the MPTP model of Parkinson's disease".Journal of Neural Transmission.108(11): 1263–82.doi:10.1007/s007020100004.PMID11768626.S2CID2834254.
- ^Langston JW, Forno LS, Rebert CS, Irwin I (February 1984). "Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey".Brain Research.292(2): 390–4.doi:10.1016/0006-8993(84)90777-7.PMID6607092.S2CID34183578.
- ^Blanchet PJ, Calon F, Morissette M, Hadj Tahar A, Bélanger N, Samadi P, Grondin R, Grégoire L, Meltzer L, Di Paolo T, Bédard PJ (July 2004). "Relevance of the MPTP primate model in the study of dyskinesia priming mechanisms".Parkinsonism & Related Disorders.10(5): 297–304.doi:10.1016/j.parkreldis.2004.02.011.PMID15196509.
- ^Tubbs RS, Loukas M, Shoja MM, Mortazavi MM, Cohen-Gadol AA (July 2011)."Félix Vicq d'Azyr (1746-1794): early founder of neuroanatomy and royal French physician".Child's Nervous System.27(7): 1031–4.doi:10.1007/s00381-011-1424-y.PMID21445631.
- ^Swanson LW (2014).Neuroanatomical terminology: a lexicon of classical origins and historical foundations.England: Oxford University Press.ISBN9780195340624.
- ^Sano T (1910)."Beitrag zur vergleichenden Anatomie der Substantia nigra, des Corpus Luysii und der Zona incerta".MSCHR Psychiat Neurol.28(1): 26–34.doi:10.1159/000209678(inactive 22 June 2024).
{{cite journal}}:CS1 maint: DOI inactive as of June 2024 (link) - ^Hornykiewicz, O. (2006). "The discovery of dopamine deficiency in the parkinsonian brain". In Riederer, P.; Reichmann, H.; Youdim, M. B. H.; Gerlach, M. (eds.).Parkinson's Disease and Related Disorders.Springer Vienna. pp. 9–15.doi:10.1007/978-3-211-45295-0_3.ISBN978-3-211-28927-3.PMID17017502.
{{cite book}}:|journal=ignored (help)