Sulfoxone
Appearance
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokineticdata | |
| Protein binding | 69% |
| Metabolism | Hepatic |
| Eliminationhalf-life | 3 to 8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
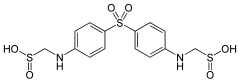
| Formula | C14H16N2Na2O6S3 |
| Molar mass | 450.45g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sulfoxoneoraldesulfone sodiumis an anti-leprosydrug.[1]It is also known asdiasone.Sulfoxone sodium was introduced in Japan in 1948.[2]Ernest Muirintroduced it to Western use while serving as superintendent of theChacachacare LeprosariumonTrinidadin theCaribbean.[3]
References
[edit]- ^"Sulfoxone".
- ^Ozawa H, Maruyama Y (2002). "[A 50-year history of new drugs in Japan: the developments of antileprosy drugs and their epidemiological aspects]".Yakushigaku Zasshi.37(1): 76–83.PMID12412600.
- ^Browne, Stanley George (1974),"Ernest Muir, C.M.G., C.I.E., M.D. (Edin.), F.R.C.S., LL.D. 1880–1974"(PDF),International Journal of Leprosy,vol. 42, no. 4,Bauru:International Leprosy Association, pp. 457–458,PMID4617724.
