TRPV6
| TRPV6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | TRPV6,ABP/ZF, CAT1, CATL, ECAC2, HSA277909, LP6728, ZFAB, transient receptor potential cation channel subfamily V member 6, HRPTTN | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM:606680;MGI:1927259;HomoloGene:56812;GeneCards:TRPV6;OMA:TRPV6 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
TRPV6is a membranecalcium (Ca2+) channelprotein which is particularly involved in the first step inCa2+absorptionin theintestine.
Classification[edit]
Transient Receptor Potential Vanilloid subfamily member 6 (TRPV6) is an epithelialCa2+channelthat belongs to thetransient receptor potential family(TRP) of proteins.[5]The TRP family is a group ofchannel proteinscritical for ionic homeostasis and the perception of various physical and chemical stimuli. TRP channels can detecttemperature,osmotic pressure,olfaction,taste,and mechanical forces.[5][6]Thehuman genomeencodes for 28 TRP channels, which include sixTRPVchannels.[5]The high Ca2+-selectivity ofTRPV5and TRPV6 makes these channels distinct from the other four TRPV channels (TRPV1-TRPV4).[7]TRPV5 and TRPV6 are involved in Ca2+transport, whereasTRPV1throughTRPV3are heat sensors with different temperature threshold for activation, andTRPV4is involved in sensingosmolarity.[8][9]Genetic defects in TRPV6 gene are linked to transient neonatal hyperparathyroidism and early-onsetchronic pancreatitis.Dysregulation of TRPV6 is also involved inhypercalciuria,kidney stone formation,bone disorders, defects inkeratinocytedifferentiation, skeletal deformities,osteoarthritis,male sterility,Pendred syndrome,and certain sub-types ofCancer.[8][9]
Identification[edit]
Penget alidentified TRPV6 in 1999 from ratduodenumin an effort to search for Ca2+transporting proteins involved in Ca2+absorption.[10]TRPV6 was also called calcium transport protein 1 (CaT1)[10][11]initially although the names epithelial calcium channel 2 (ECaC2)[12][13]and CaT1-like (CaT-L)[14]were also used in early studies to describe the channel.[10][12][13][14]The human and mouseorthologsof TRPV6 were cloned by Peng et al and Weber et al, respectively.[11][12]The name TRPV6 was confirmed in 2005.[15]
Gene location, chromosomal location, and phylogeny[edit]
The humanTRPV6geneis located onchromosomallocus7q33-34 close to its homologTRPV5on 7q35.[16][17]TheTRPV6gene in human encodes for 2906 bp-longmRNA.[17]In contrast to most other proteins, which initiate translation with an AUGcodon,TRPV6 translation is initiated by non-AUG-codon-mediated reading.[18]TRPV6 protein bears a 40-a.a-long N-terminal extension inplacentaand in some physiological settings in comparison to the annotated version of the protein used in biological studies.[18]However, it is still to be determined whether the long version of the TRPV6 protein is the dominant form in different tissues.
| Species | Human | Rat | Mouse |
| Chromosomal location | 7q33-q34 | 4q22 | 6B2 |
| Annotated aa length | 725 | 727 | 727 |
| In vivoaa lengtha | 765 | 767 | 767 |
| RefSeq nucleotide | NM_018646 | NM_053686 | NM_022413 |
| RefSeq protein | NP_061116 | NP_446138 | NP_071858 |
aTo be verified in different tissues.
It has been hypothesized thatTrpv5andTrpv6genes were generated from a single ancestral gene bygene duplicationevents.[16][19]Phylogeneticanalysis has shown that TRPV6paralogsinmammals,sauropsids,amphibians,andchondrichthyesarose out of independent duplication events in the ancestor of each group.[19]It is speculated that two specialized Ca2+-selectiveTrpvhomologs arose as an adaptation to achieve a greater degree of functional specialization for navigating distinct renal challenges of terrestrial animals.[19]
Twoallelesof theTRPV6gene have been identified in humans (originally noted as CaT-La and CaT-Lb).[14]These alleles exhibit coupledpolymorphismsgenerating two versions of the same gene.[14][20]The polymorphisms give rise to an “ancestral variant” and a “derived variant” that differ in five bases and three amino acids.[14][20]The ancestral allele codes for C197(157, in parentheses are annotated amino acid numbering), M418(378), and M721(681) whereas the derived allele codes for R197(157), V418 (378) and T721(681).[20][21]The frequency of the ancestralTRPV6allele varies across different population groups.[20][21]It is hypothesized that selection pressures that could have changedTRPV6allele distribution include changes in patterns of milk consumption, domestication of animals, change inultraviolet lightexposure due to trans-equatorial migration, genomic adaptations providing immune advantages to populations encountering new pathogens.[20][21][22]
Tissue distribution[edit]
The TRPV6 protein is expressed inepithelialtissues such as theintestine,kidney,placenta,epididymis,andexocrine glandssuch aspancreas,prostateandsalivary,sweat,andmammary glands.[23][24]TRPV6 protein expression in humans has been demonstrated in theesophagus,stomach,small intestine,colon,pancreas, mammary glands,ovary,thyroid,and prostate byimmunohistochemistryapproaches.[23]TRPV6 expression mainly confines on the apical membrane of epithelial cells. In the intestine, the protein is expressed on the brush-border membrane ofenterocyte.
Differences in the TRPV6 expression profile have been reported possibly due to variation in assay-dependent suchprimer design,hybridization probes,PCRvs.northern blotting,semi-quantitative PCR vs.RT-PCR,andantibodiesused for immunodetection.[25]TRPV6 expression profile is also influenced by age, gender, Ca2+and vitamin D3levels in food, hormonal status, location within the tissue, cellular location, reproductive status, and weaning status (see SectionRegulation).
In humans, TRPV6 transcripts have been detected in the placenta, pancreas, prostate cancer, and duodenum and the prostate by northern blotting; and in duodenum, jejunum, placenta, pancreas, testis, kidney, brain, and colon by semi-quantitative PCR.[13]In rodents, TRPV6 expression has been validated in the duodenum, cecum, small intestine, colon, placenta, pancreas, prostate, and epididymis by Northern Blotting.[10][17][26]In mouse, TRPV6 transcript abundance measured by RT-PCR is as follows: prostate > stomach, brain > lung > duodenum, cecum, heart, kidney, bone > colon > skeletal muscle > pancreas.[27]
Data from Human Protein Atlas and RNA-Seq based suggest TRPV6 mRNA is low in most tissues except for the placenta, salivary gland, pancreas, and prostate.[24][28]TRPV6 mRNA is expressed in the apical domain of murine osteoclasts of cortical bone.[29][30]Cortical and trabecular osteocytes do not express TRPV6 mRNA whereas osteoblasts show weak expression.[31]
Structure and biophysical properties[edit]
Primary and secondary structure[edit]
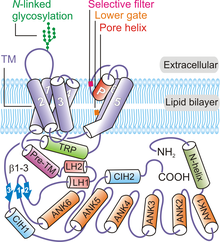
Overall, four subunits of TRPV6 arrange to form a tetrameric channel displaying a four-foldsymmetry.[8][32]Beginning fromN-terminusand moving towards theC-terminusof the protein, each TRPV6polypeptidecontains: an N-terminal helix, anankyrin repeatdomain (ARD) containing six ankyrin repeats, aβ-hairpinstructure linker domain made up twoβ-strands,a helix-turn-helix motif, a pre-SI helix, TM domain made up of six TM helices (S1 through S6), a pore-loop (also called P-loop),amphipathicTRP helix, C-terminal hook, and a six-residue β-strand (β3) (Figure 1).[8][32]
Tertiary and quaternary structure[edit]

The TRPV6 channel protein displays four-fold symmetry and contains two main compartments: a 30 Å-tall transmembrane domain with a central ion channel pore and a ~70 Å-tall and a ~110 Å-wide intracellular skirt enclosing a 50 Å × 50 Å cavity wide cavity underneath the ion channel.[32]The clustering of four TRPV6 subunits forms an aqueous pore exhibiting a fourfold symmetry (Figure 2). A pre-SI helix links the intracellular portion of the protein to the TM domain through a linker domain made up of β-hairpin structure and a helix-turn-helix motif. Helices S1 through S4 form a transmembrane helical bundle or TM domain that is inserted almost perpendicularly to the plane of theplasma membrane.[32]
The pore module elements are made up of S5, S6, and the P-loop in TM domains.[32]The pore module from each TRPV6 polypeptide participates in inter-subunit interactions to form a central ion pore (Figure 1).[32]The pore-forming elements of each TRPV6 subunit also interact with S1-S4 domains of the adjacent polypeptide in a domain-swapped arrangement.[32][33]Intersubunit interactions also occur between S1-S2 extracellular loops and S5-P and S6-P loops of the neighboring TRPV6 subunits.[32]The conservedN-linked glycosylationsite on the S1-S2 loop is required for by theKlotho-mediated activation.[34]The intracellular skirt portion of the TRPV6 protein is mainly made up of the ankyrin repeats.[32]The TRP domain is oriented parallel to the membrane and participates inhydrophobicinteractions with the TM domain and thehydrophilicinteractions in the intracellular skirt. The N-terminal helix, C-terminal hook, andβ-sheets(formed by the β-hairpin structure in the linker domain) in the channel participates in intersubunit interactions with the ARDs to provides a framework for holding the elements of the intracellular skirt together.[8][32]
Pore architecture and cation binding sites[edit]
The TRPV6 pore has four main elements, namely, the extracellular vestibule, a selectivity filter, a hydrophobic cavity, and a lower gate.[32][35][36]Facing the central lumen of the channel, a four-residue selectivity filter (538TIID541) containing fourAspartate541 (D541) side chains (one from each protomer) is critical for Ca2+selectivity and other biophysical properties of the channel.[32][35][36]This filter forms a negatively charged ring that discriminates between ions based on their size and charge.Mutationsin the critical pore-forming residue of TRPV6 blocks Ca2+uptake, a strategy has been used to generate TRPV6 loss-of-function models to examine the role of the channel in animal physiology.[35][36]Four different types of cation binding sites are thought to exist in the TRPV6 channel.[32]Site 1 is located in the central pore and shares the same plane that is occupied by the key selective residues D541. Site 2 is thought to be present about 6-8 Å below Site 1 followed by Site 3 which is located in the central pore axis about 6.8 Å below Site 2. Site 2 and 3 are thought to interact with partially-hydrated to equatorially-hydrated Ca2+ions. Finally, four symmetrical cation binding sites in the extracellular vestibule mediate the recruitment of cations towards the extracellular vestibule of TRPV6 and are referred to as recruitment sites.[32]
Ion permeation[edit]
The conductance of TRPV6 fordivalentcationsfollows the preference: Ca2+> Ba2+> Sr2+> Mn2.Intra-cellular Mg2+inhibits TRPV6 and contributes to the strong inward rectification exhibited by the channel.[37]TRPV6 uptake activity is inhibited by divalent Pb2,Cu2+,Cd2+,Zn2,Co2+,Fe2+,and trivalent cations La3+,Fe3+,Gd3+.The concentration of ions to achieve the inhibition ranges from 1 to 10 μM.[38]The TRPV6 protein is constitutive with a single-channel conductance of 42-58 ps.[7][39]At low Ca2+concentrations, a single Ca2+ion binds in the selectivity filter formed by D541 and permits Na+permeation. At high Ca2+concentration, Ca2+permeation occurs by a knock-off mechanism that involves the formation of short-lived conformations involving binding of three Ca2+ions to residue D541.[39]
Channel gating[edit]

The conformational changes involved in channel opening are hinged around the residueAlanine566 (A566) and occur in the pore-lining helix S6 (Figure 3).[39]The upper portion of S6 helix undergoes an α-to-π helical transition which forces the lower portion of the helix to turn by 100 degrees and tilt away from the pore axis by 11 degrees.[39]This conformational change moves the lower portion of the helix gating the pore and thereby widens the pore size. The conformational change alters the residues facing the pore axis and triggers the formation of newelectrostatic bondssubunit andsalt bridgesthat offset the high energetic cost of unfavorable α-to-π helical transition that occurs during channel opening.[39]
Regulation by phosphatidylinositol 4,5-bisphosphate (PIP2) and calmodulin (CaM)[edit]
The influx of Ca2+inside the cell triggers negative feedback mechanisms to suppress TRPV6 activity and prevent Ca2+overload.[9]TRPV6 channel activity is regulated by the intracellular level ofphospholipidphosphatidylinositol 4,5-bisphosphate(PIP2) and interactions with Ca2+-Calmodulin(CaM) complex.[9]The depletion of PIP2or CaM-binding inactivates TRPV6.[40][41][42][43][44]The influx of Ca2+in TRPV6 expressing cells activatesphospholipase C(PLC) which in turn hydrolyzes PIP2.Depletion in PIP2levels results in a decline in channel activity since most TRP channels require this lipid for activation.[40][43][44]The lipid PIP2can override Ca2+-CaM-mediated inhibition of TRPV6. Overall, TRPV6 inactivation by calmodulin is orchestrated by a balance of intracellular Ca2+and PIP2concentration.[40][41][42][43][44]
Interacting proteins[edit]
Among 20+ TRPV6 interactors identified so far, the functional consequences of Ca2+-binding protein Calmodulin (CaM) and Glucuronidase Klotho have been most extensively characterized [36, 37, 41, 42].[34][40][41][45][46]Functional consequences of TRPV6 channel activation are summarized in the table below).[47]
| Interactor | Consequence |
| BSPRY | N/A |
| Calbindin-D28k | N/A |
| Calmodulin | Inhibition |
| Cyclophilin B | Activation |
| FYN | PO4lyation |
| I-MFA | N/A |
| Klotho | Activation, Glycosylation (Asn-357) |
| NHERF4 | Activation |
| NIPSNAP1 | Inhibition |
| NUMB | Inhibition |
| PTEN | N/A |
| PTP1B | DePO4lyation
(Tyr-161 and Tyr-162) |
| RAB11A | Activation,
Increase in Plasma membrane level |
| RGS2 | N/A |
| RYR1 | N/A |
| S100A10 | Activation,
Increase in Plasma membrane level |
| SRC | PO4lyation (Tyr-161, 162) |
| TRPC1 | Retains in ER, Inhibition |
| TRPML3 | N/A |
| TRPV5 | Tetramer formation,
New Channel creation |
Abbreviations
Protein Interactor
BSPRY: B-Box and Spry Domain Containing Protein; FYN: Fyn Kinase Belonging Src Family of Kinases; I-MFA: Myo D Family Inhibitor; NHERF: Na Exchanger Regulatory Factor; NIPSNAP14-Nitrophenylphosphatase Domain and Non-Neuronal SNAP25-Like Protein Homolog 1; Numb: Drosophila mutation that removes most of the sensory neurons in the developing peripheral nervous system; PTP: Protein Tyrosine Phosphatase; Rab11a: Member RAS Oncogene Family; RGS2: Regulator Of G-Protein Signaling 2; RyR1: Ryanodine Receptor 1; TRPC1: Transient receptor potential canonical 1; TRPML3: Transient receptor potential Mucolipin-3.
Physiological functions[edit]
The Ca2+-selective channel proteins TRPV6 and TRPV5 cooperate to maintain calcium concentration in specific organs.[22][48]TRPV6 functions as apical Ca2+entry channels mediatingtranscellular transportof this ion in the intestine, placenta, and possibly some other exocrine organs. TRPV6 also plays important roles in maternal-fetal calcium transport,[49]keratinocyte differentiation,[50]and Ca2+homeostasis in the endolymphatic system of thevestibularsystem,[51][52]and maintenance of male fertility.[53][54]
Ca2+absorption in intestine[edit]
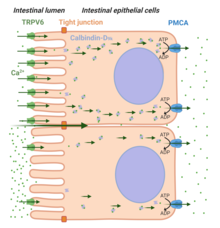
Two routes of Ca2+absorption are recognized:paracellular transportandtranscellular transport(seeFigure 4).[55]A high-Ca2+-diet favors paracellular transport of the ion across the length of the intestine allowing them to pass between the intercellulartight junctionsthat connect epithelial cells. In contrast under conditions when [Ca2+] in the lumen of the intestine is lower in comparison to its concentration in theplasma(e.g. during low dietary Ca2+), the transcellular pathway is required for adequate Ca2+absorption. Three important steps in transcellular Ca2+transport are recognized: cellular entry of Ca2+ion on the apical side via TRPV6 (Step-1), the binding of Ca2+ion withcalbindin-D9k(Step-2),and exit of Ca2+from the basolateral side via theplasma membrane Ca2+ATPase(PMCA1b).[55]The hormoneVitamin D3(or 1,25(OH)2D3) plays an important role in TRPV6-mediated intestinal Ca2+absorption).[55]
Ca2+reabsorption in the kidney[edit]
In contrast to the intestine, where TRPV6 is the gatekeeper of Ca2+absorption, the transcellular reabsorption of this ion in the kidney occurs through TRPV5. Although TRPV5 is a recognized gatekeeper for transcellular reabsorption of Ca2+ion in the kidney, TRPV6knockout(KO) mice also struggle to concentrate their urine and display hypercalciuria.[56]TRPV6 is known to co-localize with TRPV5 Calbindin-D28Kin apical domains of distal convoluted tubules and connecting tubules [20]. TRPV5 KO mice compensate for Ca2+loss by increasing TRPV6 expression in the duodenum.[56]Moreover, a recent study analyzing vitamin D responsive genes inovine,canineand,equinekidney suggested that TRPV6, calD9k/calD28k,and PMCA could be the main pathways orchestrating transcellular Ca2+transport in the kidney of sheep, dogs, and horses.[57]
Maternal-fetal Ca2+transport[edit]
TRPV6 plays an indispensable role in placental Ca2+transport.[49]Fetalbone mineralizationpeaks during late pregnancy. At this stage, fetal blood has a higher concentration of Ca2+in comparison to maternal blood thereby creating conditions that require active transcellular transport of Ca2+from mother to the fetus.[58][59]This process is very important since defects in placental transport of calcium can be precursors for Ca2+deficiency syndromes andintrauterine growth restrictions.[60]The expression of TRPV6 increases 14-fold during the last 4 days of themurinegestationalperiod and coincides with the peak phase of fetal bone mineralization.[49]The protein TRPV6 is abundantly expressed in the mammalian placental tissues.[49][61][62][63][64]Indeed, TRPV6 expression is ~1000-fold higher in comparison to TRPV5. In the placenta, TRPV6 is expressed introphoblastsandsyncytiotrophoblasts.[14][61]In mice, TRPV6 mRNA and protein are expressed in the intraplacentalyolk sacand the visceral layer of the extraplacental yolk sac.[49]Most importantly, TRPV6 KO fetuses exhibit a 40% reduction in45Ca2+transport activity and a dramatic decrease in the ash weight (a measure of fetal bone health).[49]In humans, trophoblasts fluid shear stress (FSS) is known to induce a TRPV6-mediated Ca2+influx and promotemicrovilliformation through a mechanism involving Ezrin and Akt-phosphorylation.[65]
Epididymal Ca2+regulation and implications on male fertility[edit]
The regulation of calcium concentration in theepididymallumen is critical forsperm motility.[66]TRPV6-mediated reduction of luminal Ca2+concentration in the epididymis is critical for male fertility in mice.[53]TRPV6 KO mice or mice expressing loss-of-function version of TRPV6 channel (Trpv6D541Ahomozygousmice) have a severely impairedfertility.[53]Mice expressing nonfunctional TRPV6 have a 10-fold higher concentration of Ca2+in the epididymal lumen and Ca2+uptake in this space is reduced by 7-to-8 folds.[53][54]The increases Ca2+ion in epididymal lumen concentration leads to significant defects in motility, fertilization capacity, and viability of sperms inTRPV6D541Amice.[53][54]It appears TRPV6 and chloride channel transmembrane manner 16 A (TMEM16A) act cooperatively to reduce the luminal concentration of Ca2+in the epididymal lumen.[67]
Bone health[edit]
Under conditions of sub-optimal dietary Ca2+,normal serum calcium levels in TRPV6 KO mice are maintained at the expense of bone.[68][69]TRPV6 plays an important role in osteoclasts but not in osteoblasts.[68][69]In mice, TRPV6 depletion results in increased osteoclasts differentiation[29]whereas TRPV5 is essential for proper osteoclastic bone resorption.[68]
Keratinocyte differentiation[edit]
Keratinocytes differentiation is orchestrated by calcium switch, a process that entails an influx of Ca2+in keratinocyte which induces broad transcriptional changes necessary fordesmosomeformation, stratification, and cornification.[70]TRPV6 KO mice display thinner layers ofstratum corneumand 20% of the mice also showalopeciaanddermatitis.[56]The silencing of TRPV6 impairs Ca2+-mediated differentiation of human primary keratinocytes and downregulates differentiation markers such asinvolucrin,transglutaminase-1,andcytokeratin-10.The hormone1,25-dihydroxyvitamin-D3upregulates TRPV6 in keratinocytes and triggers a Ca2+influx. This in turn induces the expression of keratinocyte differentiation-specific pathways.[50]
Role in the inner ear[edit]
The proteins TRPV5 and TRPV6 are expressed in several regions of theinner earas well as in primary cultures of semicircular canal duct (SCCD) epithelium.[51][52]Some studies have indicated that TRPV5 and TRPV6 are needed for lowering the Ca2+concentration in the lumen of mammalianendolymph,a requirement that is essential for normalhearingand balance.[51][52][71]
Uterine and placental expression of TRPV6 and implications in pregnancy[edit]
Theendometrialanduterineexpression of TRPV6 has been reported in mammals.[72][73][74]The expression of TRPV6 in the uterus is thought to be hormonally regulated by17β-estradiolandprogesteronein rodents. In rodents, TRPV6 mRNA is expressed in the labyrinth and spongy zone as well as placenta-unattached areas of the uterus. The stage of pregnancy is an important regulator of TRPV6 expression. The downregulation of TRPV5/6 expression and a resulting decline in Ca2+transport is thought to change the proliferative profile of human trophoblasts; a process which in turn is linked to the development ofpre-eclampsia.[73]This juxtaposition of TRPV6 expression and its stringent regulation bysex hormonesduring pregnancy suggest that the protein may be important forembryo implantation,however conclusive evidence for this connection does not exist.[72][73][74]
Implications in Human Diseases[edit]
Transient Neonatal Hyperparathyroidism[edit]
Loss of TRPV6 in murine placenta severely impairs Ca2+transport across trophoblast and reduces embryo growth, induces bone calcification, and impairs bone development. In humans, the insufficient maternal-fetal transport caused by pathogenic genomic variants of TRPV6 is thought to be a cause for skeletal defects observed in selected case reports oftransient neonatal hyperparathyroidism(TNHP) cases. These variants are believed to compromise the plasma membrane localization of the protein.Exome sequencingof an infant with severeantenatalonsetthoracic insufficiencywith accompanying fetal skeletal abnormalities indicates the critical role of TRPV6 in maternal-fetal transport. The study indicated that compoundheterozygousvariants of TRPV6 result in severe undermineralization and severedysplasiaof the fetal skeleton.[75][76][77]
Chronic Pancreatitis[edit]
Recent evidence indicates that naturally occurring TRPV6 loss of function variants predisposes certaindemographicstochronic pancreatitis(CP) by dysregulating calcium homeostasis in the pancreatic cells.[78][79]Sequencingstudies among chronic pancreatitis patients revealed the presence of 33missenseand 2nonsensevariants predisposedJapanese,German,andFrenchpatients to a higher risk of CP.[79]Overall, these studies have shown that disease-inducing TRPV6 loss-of-function genomic variants are over-represented in German, French,Chinese,and Japanese CP patients in comparison to controls in their respective groups.[78][79]The loss-of-function variants are believed to compromise calcium transport in the pancreas by act by either reducing the total protein level and/or compromising Ca2+uptake activity by the channel.[79]
Kidney Stone Formation[edit]
The role of TRPV6 in renal stone formation has been suggested through sequencing studies conducted on acohortof 170 patients inSwitzerland.[80]The studies revealed that the frequency of TRPV6 gain-of-function haplotype is significantly higher in Ca2+-stone formers (nephrolithiasis) in comparison to non-formers. The observed hypercalciuriaphenotypesfrom animal studies and studies on TRPV6single nucleotide polymorphisms(SNPs) suggest that TRPV6 haplotype could be an important risk factor for absorptive and renal hypercalciuria (kidney stones due to impaired intestinal absorption and renal re-absorption respectively). The lower incidence of kidney stone diseases inAfrican-Americansand a relatively higher prevalence of ancestral haplotype suggest theory according to which this haplotype endows an advantage of increased Ca2+reabsorption in this demographic and reduces the incidence of kidney stones.[14][20][22][80]
Bone Resorptive Diseases[edit]
TRPV6 KO mice exhibit osteoporosis-like symptoms such as reducedbone mineral densityand hypercalciuria.[56]The hormoneestrogen,the deficiency of which is linked topost-menopausal osteoporosis,also regulates the expression of TRPV6 in humans. Indeed, a lower calcium absorption seen in olderpostmenopausalwomen is attributed to reduced TRPV6.[81]The C-terminal portion ofSoricidinis a drug that inhibits Ca2+-uptake activity by binding to TRPV6.Preclinicalstudies of this drug have shown great promise in the treatment of bone resorptive diseases.[28]
The high degree of similarity between Hereditary Vitamin D–Resistant Rickets (HVDRR) disease symptoms and observed phenotypes in TRPV6 KO mice has led some experts to postulate pathological connections between the disease and TRPV6 dysfunction.[48]TRPV6 plays an important chondroprotective role by regulating multiple aspects ofchondrocytefunction, such asextracellular matrixsecretion, the release of matrix-degrading enzymes,cell proliferation,andapoptosis.[82]Furthermore, TRPV6 knockout mice display multipleosteoarthritis(OA) phenotypes such ascartilagefibrillation,eburnation,and loss ofproteoglycans.[82]
Pendred Syndrome[edit]
The dysfunction geneSlc26a4has been linked toPendred syndrome– a genetic disorder that results insyndromic deafnessin children.[71][83]The disease is caused by mutations in which compromise the function of the encoded protein pendrin - an anionCl−/HCO3−exchangerexpressed in the inner ear.[71][83]The loss of function in this gene is thought to reduce thepHvalue of mammalian endolymph and impair Ca2+absorption via TRPV5 and TRPV6.[83]This in turn could prevent the uptake of Ca2+and impairs the luminal reduction in Ca2+concentration within the endolymphatic system of the ear.[71][83]
Cancer[edit]
Theoverexpressionof TRPV6 has been validated in the colon,parathyroid,pancreatic, andthyroid cancer[23]whereas its expression is reportedlydownregulatedinesophageal cancer,[84]non-small cell lung cancer,[85]andrenal cancer.[86]TRPV6 is considered to be an oncochannel that is hypothesized to mediate cancer progression by triggering Ca2+-entry induced aberrations in molecular drivers regulating processes such ascell cycle,apoptosis,andmigration;thereby conferring proliferative and survival advantages to cancer cells.[25][28][87]Overexpression of TRPV6 correlates strongly withpathological stage,tumor grade,extra-prostatic invasion,lymph nodemetastasis,and resistance toandrogen-targeted therapies inprostate cancer.[14][23][87][88]The expression TRPV6 has been touted as a prognostic marker for advanced prostate cancer since its expression is strongly dependent on the grade of the tumor.[87][88]Expression of TRPV6 is significantly elevated in breastadenocarcinomatissue in comparison to normal breast tissue.[89][90]TRPV6 expression has been reported multiple breastcancer cell linesand prostate cancer cell lines.[87][91][92][93]The prostate cancer cell lines PC-3 and LnCAP overexpress TRPV6 relative to benign epithelial cells PrEC and BPH-1.[87]The silencing of TRPV6 in prostate cancer cells decreases proliferation rate,S-phaseaccumulation, and expression oftumor markerproliferating cell nuclear antigen(PCNA) expression.[92]TRPV6 overexpression is believed to induce aberrant Ca2+-uptake in prostate cancer line and activate transcription factorNuclear Factor of Activated T cells(NFAT).[92]
Expression of TRPV6 is upregulated by estrogen, progesterone, and estradiol in breast cancer cell lineT47D.[90]In agreement with these observations, theestrogen receptorantagonistTamoxifenreduces TRPV6 expression in T47D cells and suppresses Ca2+-uptake of the channel in bothER-positiveandER-negativebreast cancer cell lines.[94]The overexpression of TRPV6 is associated with early-stagecolon cancerand its silencing in colon cancer induces apoptosis and inhibits cancer cell proliferation.[95]In terms of mechanism, mutations within the calmodulin-binding domains of TRPV6 channels confers invasive properties to colon adenocarcinoma cells.[96]The proteinsp38αandGADD45αare believed to upregulate TRPV6 expression signaling in SW480 colon cancer cells by enhancing vitamin D signaling.[97]TRPV6 has been reported to amplifyInsulin-like growth factors(IGF)-inducedPI3K-PDK1-Aktsignaling in human colon cancer and promote colon cancer.[98]
TRPV6 is up-regulated in primary cancer tissues frompancreatic cancerpatients and promotes the proliferation of pancreaticneuroendocrine tumorsNFAT-dependent mechanisms.[99]Silencing of TRPV6 induces apoptosis and cell cycle arrest in pancreatic cancer cells and inhibits their invasion, proliferation, and migration.[100]Forced expression of TRPV6 ingastric cancercells increases their sensitivity tocapsaicin-induced apoptosis whereas thesiRNA-mediated silencing of the channel suppresses this sensitivity.[101]TRPV6 downregulation inesophageal carcinomahas been suggested to be a prognostic marker ofdisease-specific survivalin patients suffering fromesophageal cancer.[102]Low TRPV5 and TRPV6 co-expression have suggested as predictive markers for poor recurrence-free survival innon-small cell lung cancer.[85]
Pharmacological Targeting[edit]
Several chemical inhibitors are known to inhibit TRPV6. Some compounds that have demonstrated inhibitory activity towards TRPV6 include TH-1177,2-Aminoethoxydiphenyl borate(2-APB), 2-APB derivative 22b,Econazole,Miconazole,Piperazinederivative Cis-22a,Capsaicin,Δ9-tetrahydrocannabivarin,Xestospongin C,Lidocaine,gold-caged nanoparticle (PTX-PP@Au NPs) andSorcidinC-13 (SOR-C13) synthetic peptide.[28]Among different inhibition strategies tested so far, the 13-amino acid peptide SOR-C13 has shown the most promise. This 13-amino acid peptide derived from 54-amino acid peptide found in the paralytic venom of thenorthern short-tailed shrew(Blarina brevicauda) reduces cancer growth in cell and animal models. This anti-cancer agent has recently completed aPhase Iclinical safety trial that had enrolled 23 patients with advancedsolid tumorsof epithelial origin non-responsive to allstandard-of-caretreatments.[28]
Regulation[edit]
The regulation of TRPV6 can be examined mainly in the context of its physiological, hormonal, and molecular factors.[22]The hormonal regulation of TRPV6 has been characterized most extensively. In this regard, its regulation by the hormone vitamin D3and sex hormones has been examined in considerable detail. Rodent studies suggest that the TRPV6 channel is regulated by a wide range of physiological factors such as diet, age, gender, pregnancy, lactation, sex hormones, exercise, age, and gender. Some biological and pharmacological agents known to regulate TRPV6 includeglucocorticoids,immunosuppressive drugs,anddiuretics.[22]
Vitamin D[edit]
Multiple dose-response and time-course experiments in rodents and colon cancer cell lines have conclusively shown TRPV6 mRNA is robustly induced by this vitamin D at extremely low concentrations.[103][104]At least fivevitamin D response elements(VDREs) at positions −1.2, −2.1, −3.5, −4.3, and −5.5 kb relative to transcriptional start site (TSS) have been identified on TRPV6 transcripts.[105]Among these five sites, VDREs at positions −1.2, −2.1, and −4.3 kb are significantly more responsive to1,25-(OH)2D3in comparison to VDREs located at −3.5 and −5.5 kb which do not appear to contribute substantially to vitamin D mediated transcriptional regulation in the intestine.[105]Mechanism wise, TRPV6 transcription is initiated in response tovitamin D Receptor(VDR)-mediated signaling, although other non-direct mechanisms cannot be ruled out.[104][106]Important steps in vitamin D mediated transcriptional regulation include 1) binding of vitamin D on its cognate vitamin D receptor (VDR), 2) the translocation of vitamin D receptor (VDR)-retinoid X receptorheterodimer complex in thenucleus,3) binding VDR-RXR complex on the TRPV6gene promoter,4) recruitment ofsteroid receptor coactivator 1andRNA polymerase IIon the promoter, and 5) transcriptional activation mediated throughhistone H4acetylationevents.[107]
Diet[edit]
The level ofCa2+and vitamin D in the diet are the most important regulators of TRPV6 expression.[104]The expression of TRPV6 is thought to be modulated strongly to fine-tune Ca2+absorption from the diet, especially under conditions when dietary Ca2+availability is low.[103][104]In rodents, restricting Ca2+availability in the diet induces dramatic up-regulation in the duodenal expression of TRPV6.[103][104]Calcium influx from the diet and its subsequent binding to calbindin-D9kcould be the rate-limiting step that modulates vitamin D-dependent regulation TRPV6.[108]When dietary Ca2+is insufficient, normal blood calcium levels in TRPV6 KO mice are maintained at the expense of bone.[68][69]In many rodent lines, genetic variations in TRPV6, calbindin-D9k,PMCA1b mRNA influence intestinal Ca absorption and its impact on bone marrow density.[109]
Pregnancy and lactation[edit]
Duodenal expression of TRPV6 transcripts is upregulated in WT and VDR KO mice duringpregnancyandlactation.[110]The hormoneprolactinupregulates TRPV6 transcription and facilitates an increase in intestinal Ca2+absorption in lactating and pregnant rats, possibly as an adaptive mechanism for overcoming the loss in bone mineralization content during lactation.[111]
Aging[edit]
The intestinal expression of TRPV6 in mice varies dramatically by age and relative tissue location.[112]The duodenal expression of TRPV6 is undetectable at P1 and increases 6-fold as mice age to P14. Similarly, the expression also varies with age in thejejunum,where TRPV6 levels increases from P1 to P14, become weak at 1-month age and becomes undetectable in older mice.[112]The expression of TRPV6 in older rats (12-months) is at least 50% lower in comparison to younger counterparts (2-months old).[104]In both WT and VDR KO mice, the age-associated decline in intestinal absorption of Ca2+is accompanied by a decline in duodenal expression of TRPV6.[113]
Sex hormones[edit]
Sex hormones play an important role in the regulation of TRPV6. In comparison to male mice, female mice exhibit a 2-fold higher increase in duodenal expression of TRPV6 mRNA following vitamin D treatment.[citation needed]Sex hormone-associated differential regulation of TRPV6 across genders is believed to be correlated to differences in relative risk to osteoporosis in older postmenopausal women which have been reported to have lower TRPV6 and VDR expression in comparison to males.[81]
Estrogen treatment upregulates TRPV6 transcripts by 8-fold in VDR KO mice and by 4-fold inovariectomizedmice.[106]Greater than 50% reduction in TRPV6 mRNA has been observed inestrogen receptor αKO mice.[110]It is believed that estrogen could be differentially regulating Ca2+absorption in the duodenum by increasing TRPV6 expression through ERα.[114]Anti-progesterone agentRU486and anti-estrogen agentICI 182,780suppress TRPV6 expression in rodents by their respective antagonist action on progesterone andestrogen receptors.[115]Estrogen, progesterone, anddexamethasoneare known to upregulate TRPV6 expression in thecerebral cortexandhypothalamusof mice suggesting a potential involvement of TRPV6 in calcium absorption in thebrain.[116]
Glucocorticoids[edit]
Subcutaneous administration of glucocorticoids dexamethasone induces both renal and intestinal expression of TRPV6 in mice within 24 hours of whereas oral application ofprednisolonereduction in TRPV6 which is also accompanied by reduced Ca2+absorption in duodenum.[117][118]Intestinal regulation of TRPV6 in response to glucocorticoids appears to be VDR-dependent.[117][118]The enzymeserum and glucocorticoid-regulated kinase 1(SKG1) regulates TRPV6 expression by enhancingphosphatidylinositol-3-phosphate-5-kinasePIKfyve (PIP5K3).[119]This kinase is critical for the generation of secondary messenger PIP2,a known lipid activator of TRPV6.[119]
- TRPV
- TRPV5
- calcium channels
- calcium absorption
- transcellular pathway
- gating mechanism
- calmodulin
- maternal-fetal transport
- transient neonatal hyperparathyroidism
- chronic pancreatitis
- kidney stones
- cancer
Notes[edit]
The 2020 version of this article was updated by an external expert under a dual publication model. The correspondingacademic peer reviewedarticle was published inGeneand can be cited as: Vinayak Khattar, Lingyun Wang, Ji-Bin Peng (5 April 2022). "Calcium selective channel TRPV6: Structure, function, and implications in health and disease".Gene.Gene Wiki Review Series.817.doi:10.1016/J.GENE.2022.146192.ISSN0378-1119.PMID35031425.WikidataQ110874871. |
References[edit]
- ^abcENSG00000165125 GRCh38: Ensembl release 89: ENSG00000276971, ENSG00000165125–Ensembl,May 2017
- ^abcGRCm38: Ensembl release 89: ENSMUSG00000029868–Ensembl,May 2017
- ^"Human PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^"Mouse PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^abcVenkatachalam K, Montell C (2007)."TRP channels".Annual Review of Biochemistry.76:387–417.doi:10.1146/annurev.biochem.75.103004.142819.PMC4196875.PMID17579562.
- ^Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, et al. (February 2002)."A unified nomenclature for the superfamily of TRP cation channels".Molecular Cell.9(2): 229–31.doi:10.1016/s1097-2765(02)00448-3.OCLC1106536919.PMID11864597.
- ^abYue L, Peng JB, Hediger MA, Clapham DE (April 2001). "CaT1 manifests the pore properties of the calcium-release-activated calcium channel".Nature.410(6829): 705–9.Bibcode:2001Natur.410..705Y.doi:10.1038/35070596.PMID11287959.S2CID4404582.
- ^abcdeYelshanskaya MV, Nadezhdin KD, Kurnikova MG, Sobolevsky AI (February 2020)."Structure and function of the calcium-selective TRP channel TRPV6".The Journal of Physiology.599(10): 2673–2697.doi:10.1113/JP279024.PMC7689878.PMID32073143.
- ^abcdPeng JB, Suzuki Y, Gyimesi G, Hediger MA (2018). "TRPV5 and TRPV6 Calcium-Selective Channels.".Calcium Entry Channels in Non-Excitable Cells.Methods in signal transduction series. Boca Raton, FL: CRC Press/Taylor & Francis. pp. 241–274.doi:10.1201/9781315152592-13.ISBN978-1-315-15259-2.PMID30299660.
- ^abcdPeng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA (August 1999)."Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption".The Journal of Biological Chemistry.274(32): 22739–46.doi:10.1074/jbc.274.32.22739.PMID10428857.S2CID23616713.
- ^abPeng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, et al. (November 2000). "Human calcium transport protein CaT1".Biochemical and Biophysical Research Communications.278(2): 326–32.doi:10.1006/bbrc.2000.3716.PMID11097838.
- ^abcWeber K, Erben RG, Rump A, Adamski J (December 2001). "Gene structure and regulation of the murine epithelial calcium channels ECaC1 and 2".Biochemical and Biophysical Research Communications.289(5): 1287–94.doi:10.1006/bbrc.2001.6121.PMID11741335.
- ^abcHoenderop JG, Vennekens R, Müller D, Prenen J, Droogmans G, Bindels RJ, Nilius B (December 2001)."Function and expression of the epithelial Ca(2+) channel family: comparison of mammalian ECaC1 and 2".The Journal of Physiology.537(Pt 3): 747–61.doi:10.1113/jphysiol.2001.012917.PMC2278984.PMID11744752.
- ^abcdefghWissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, et al. (June 2001)."Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer".The Journal of Biological Chemistry.276(22): 19461–8.doi:10.1074/jbc.m009895200.PMID11278579.S2CID25833991.
- ^Clapham DE, Julius D, Montell C, Schultz G (December 2005). "International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels".Pharmacological Reviews.57(4): 427–50.doi:10.1124/pr.57.4.6.PMID16382100.S2CID17936350.
- ^abMüller D, Hoenderop JG, Merkx GF, van Os CH, Bindels RJ (August 2000). "Gene structure and chromosomal mapping of human epithelial calcium channel".Biochemical and Biophysical Research Communications.275(1): 47–52.doi:10.1006/bbrc.2000.3227.PMID10944439.
- ^abcFecher-Trost C, Weissgerber P, Wissenbach U (2014). "TRPV6 Channels".Mammalian Transient Receptor Potential (TRP) Cation Channels.Handbook of Experimental Pharmacology. Vol. 222. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 359–84.doi:10.1007/978-3-642-54215-2_14.ISBN978-3-642-54214-5.PMID24756713.
- ^abFecher-Trost C, Wissenbach U, Beck A, Schalkowsky P, Stoerger C, Doerr J, et al. (June 2013)."The in vivo TRPV6 protein starts at a non-AUG triplet, decoded as methionine, upstream of canonical initiation at AUG".The Journal of Biological Chemistry.288(23): 16629–44.doi:10.1074/jbc.M113.469726.PMC3675598.PMID23612980.
- ^abcFlores-Aldama L, Vandewege MW, Zavala K, Colenso CK, Gonzalez W, Brauchi SE, Opazo JC (May 2020)."Evolutionary analyses reveal independent origins of gene repertoires and structural motifs associated to fast inactivation in calcium-selective TRPV channels".Scientific Reports.10(1): 8684.Bibcode:2020NatSR..10.8684F.doi:10.1038/s41598-020-65679-6.PMC7250927.PMID32457384.
- ^abcdefAkey JM, Swanson WJ, Madeoy J, Eberle M, Shriver MD (July 2006)."TRPV6 exhibits unusual patterns of polymorphism and divergence in worldwide populations".Human Molecular Genetics.15(13): 2106–13.doi:10.1093/hmg/ddl134.PMID16717058.
- ^abcSoejima M, Tachida H, Koda Y (February 2009). "Sequence analysis of human TRPV6 suggests positive selection outside Africa".Biochemical Genetics.47(1–2): 147–53.doi:10.1007/s10528-009-9222-x.PMID19169858.S2CID39897282.
- ^abcdePeng JB (2011). "TRPV5 and TRPV6 in Transcellular Ca2+ Transport: Regulation, Gene Duplication, and Polymorphisms in African Populations".Transient Receptor Potential Channels.Advances in Experimental Medicine and Biology. Vol. 704. pp. 239–75.doi:10.1007/978-94-007-0265-3_14.ISBN978-94-007-0264-6.PMID21290300.
- ^abcdZhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR (December 2002)."Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies".Laboratory Investigation; A Journal of Technical Methods and Pathology.82(12): 1755–64.doi:10.1097/01.lab.0000043910.41414.e7.PMID12480925.S2CID6053178.
- ^ab"TRPV6 protein expression summary".The Human Protein Atlas.Retrieved2020-08-01.
- ^abLehen'kyi V, Raphaël M, Prevarskaya N (March 2012)."The role of the TRPV6 channel in cancer".The Journal of Physiology.590(6): 1369–76.doi:10.1113/jphysiol.2011.225862.PMC3382328.PMID22331416.
- ^Hirnet D, Olausson J, Fecher-Trost C, Bödding M, Nastainczyk W, Wissenbach U, et al. (May 2003). "The TRPV6 gene, cDNA and protein".Cell Calcium.33(5–6): 509–18.doi:10.1016/s0143-4160(03)00066-6.PMID12765696.
- ^Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ (November 2003)."Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney".Journal of the American Society of Nephrology.14(11): 2731–40.doi:10.1097/01.asn.0000094081.78893.e8.PMID14569082.
- ^abcdeStewart JM (2020)."TRPV6 as A Target for Cancer Therapy".Journal of Cancer.11(2): 374–387.doi:10.7150/jca.31640.PMC6930427.PMID31897233.
- ^abChen F, Ni B, Yang YO, Ye T, Chen A (2014)."Knockout of TRPV6 causes osteopenia in mice by increasing osteoclastic differentiation and activity".Cellular Physiology and Biochemistry.33(3): 796–809.doi:10.1159/000358653.PMID24686448.S2CID19539099.
- ^van der Eerden BC, Weissgerber P, Fratzl-Zelman N, Olausson J, Hoenderop JG, Schreuders-Koedam M, et al. (May 2012). "The transient receptor potential channel TRPV6 is dynamically expressed in bone cells but is not crucial for bone mineralization in mice".Journal of Cellular Physiology.227(5): 1951–9.doi:10.1002/jcp.22923.PMID21732366.S2CID7094759.
- ^Little R, Muimo R, Robson L, Harris K, Grabowski PS (2011-11-29)."The transient receptor potential ion channel TRPV6 is expressed at low levels in osteoblasts and has little role in osteoblast calcium uptake".PLOS ONE.6(11): e28166.Bibcode:2011PLoSO...628166L.doi:10.1371/journal.pone.0028166.PMC3226639.PMID22163264.
- ^abcdefghijklmnSaotome K, Singh AK, Yelshanskaya MV, Sobolevsky AI (June 2016)."Crystal structure of the epithelial calcium channel TRPV6".Nature.534(7608): 506–11.Bibcode:2016Natur.534..506S.doi:10.1038/nature17975.PMC4919205.PMID27296226.
- ^Singh AK, Saotome K, Sobolevsky AI (September 2017)."Swapping of transmembrane domains in the epithelial calcium channel TRPV6".Scientific Reports.7(1): 10669.Bibcode:2017NatSR...710669S.doi:10.1038/s41598-017-10993-9.PMC5587609.PMID28878326.
- ^abLu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG (November 2008)."The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6".Nephrology, Dialysis, Transplantation.23(11): 3397–402.doi:10.1093/ndt/gfn291.hdl:2066/70822.PMID18495742.
- ^abcOwsianik G, Talavera K, Voets T, Nilius B (January 2006). "Permeation and selectivity of TRP channels".Annual Review of Physiology.68(1): 685–717.doi:10.1146/annurev.physiol.68.040204.101406.PMID16460288.
- ^abcVoets T, Janssens A, Droogmans G, Nilius B (April 2004)."Outer pore architecture of a Ca2+-selective TRP channel".The Journal of Biological Chemistry.279(15): 15223–30.doi:10.1074/jbc.m312076200.PMID14736889.S2CID23827272.
- ^Voets T, Janssens A, Prenen J, Droogmans G, Nilius B (March 2003)."Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6".The Journal of General Physiology.121(3): 245–60.doi:10.1085/jgp.20028752.PMC2217333.PMID12601087.
- ^Kovacs G, Danko T, Bergeron MJ, Balazs B, Suzuki Y, Zsembery A, Hediger MA (January 2011). "Heavy metal cations permeate the TRPV6 epithelial cation channel".Cell Calcium.49(1): 43–55.doi:10.1016/j.ceca.2010.11.007.PMID21146870.S2CID41086787.
- ^abcdeSingh AK, Saotome K, Sobolevsky AI (September 2017)."Swapping of transmembrane domains in the epithelial calcium channel TRPV6".Scientific Reports.7(1): 10669.Bibcode:2017NatSR...710669S.bioRxiv10.1101/141523.doi:10.1038/s41598-017-10993-9.PMC5587609.PMID28878326.
- ^abcdCao C, Zakharian E, Borbiro I, Rohacs T (February 2013)."Interplay between calmodulin and phosphatidylinositol 4,5-bisphosphate in Ca2+-induced inactivation of transient receptor potential vanilloid 6 channels".The Journal of Biological Chemistry.288(8): 5278–90.doi:10.1074/jbc.M112.409482.PMC3581402.PMID23300090.
- ^abcBödding M, Flockerzi V (August 2004)."Ca2+ dependence of the Ca2+-selective TRPV6 channel".The Journal of Biological Chemistry.279(35): 36546–52.doi:10.1074/jbc.m404679200.PMID15184369.S2CID22842694.
- ^abLambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ (July 2004)."Regulation of the mouse epithelial Ca2(+) channel TRPV6 by the Ca(2+)-sensor calmodulin".The Journal of Biological Chemistry.279(28): 28855–61.doi:10.1074/jbc.m313637200.hdl:2066/57723.PMID15123711.S2CID23453339.
- ^abcZakharian E, Cao C, Rohacs T (November 2011)."Intracellular ATP supports TRPV6 activity via lipid kinases and the generation of PtdIns(4,5) P₂".FASEB Journal.25(11): 3915–28.doi:10.1096/fj.11-184630.PMC3205842.PMID21810903.
- ^abcThyagarajan B, Benn BS, Christakos S, Rohacs T (March 2009)."Phospholipase C-mediated regulation of transient receptor potential vanilloid 6 channels: implications in active intestinal Ca2+ transport".Molecular Pharmacology.75(3): 608–16.doi:10.1124/mol.108.052449.PMC2684912.PMID19073818.
- ^Derler I, Hofbauer M, Kahr H, Fritsch R, Muik M, Kepplinger K, et al. (November 2006)."Dynamic but not constitutive association of calmodulin with rat TRPV6 channels enables fine tuning of Ca2+-dependent inactivation".The Journal of Physiology.577(Pt 1): 31–44.doi:10.1113/jphysiol.2006.118661.PMC2000671.PMID16959851.
- ^Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C (March 2001)."Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin".Proceedings of the National Academy of Sciences of the United States of America.98(6): 3600–5.Bibcode:2001PNAS...98.3600N.doi:10.1073/pnas.051511398.PMC30699.PMID11248124.
- ^Shin YC, Shin SY, So I, Kwon D, Jeon JH (January 2011)."TRIP Database: a manually curated database of protein-protein interactions for mammalian TRP channels".Nucleic Acids Research.39(Database issue): D356-61.doi:10.1093/nar/gkq814.PMC3013757.PMID20851834.S2CID16278877.
- ^abSuzuki Y, Landowski CP, Hediger MA (March 2008). "Mechanisms and regulation of epithelial Ca2+ absorption in health and disease".Annual Review of Physiology.70(1): 257–71.doi:10.1146/annurev.physiol.69.031905.161003.PMID17850211.
- ^abcdefSuzuki Y, Kovacs CS, Takanaga H, Peng JB, Landowski CP, Hediger MA (August 2008)."Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport".Journal of Bone and Mineral Research.23(8): 1249–56.doi:10.1359/jbmr.080314.PMC2680174.PMID18348695.
- ^abLehen'kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, Prevarskaya N (August 2007)."TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes".The Journal of Biological Chemistry.282(31): 22582–91.doi:10.1074/jbc.m611398200.PMID17550901.S2CID22082147.
- ^abcYamauchi D, Nakaya K, Raveendran NN, Harbidge DG, Singh R, Wangemann P, Marcus DC (January 2010)."Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth".BMC Physiology.10(1): 1.doi:10.1186/1472-6793-10-1.PMC2825184.PMID20113508.S2CID5773117.
- ^abcYamauchi D, Raveendran NN, Pondugula SR, Kampalli SB, Sanneman JD, Harbidge DG, Marcus DC (June 2005). "Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal".Biochemical and Biophysical Research Communications.331(4): 1353–7.doi:10.1016/j.bbrc.2005.04.053.PMID15883024.
- ^abcdeWeissgerber P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, et al. (May 2011). "Male fertility depends on Ca²+ absorption by TRPV6 in epididymal epithelia".Science Signaling.4(171): ra27.doi:10.1126/scisignal.2001791.PMID21540454.S2CID206670887.
- ^abcWeissgerber P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, et al. (May 2012)."Excision of Trpv6 gene leads to severe defects in epididymal Ca2+ absorption and male fertility much like single D541A pore mutation".The Journal of Biological Chemistry.287(22): 17930–41.doi:10.1074/jbc.m111.328286.PMC3365704.PMID22427671.
- ^abcDiaz de Barboza G, Guizzardi S, Tolosa de Talamoni N (June 2015)."Molecular aspects of intestinal calcium absorption".World Journal of Gastroenterology.21(23): 7142–54.doi:10.3748/wjg.v21.i23.7142.PMC4476875.PMID26109800.
- ^abcdBianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, et al. (February 2007)."Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene".Journal of Bone and Mineral Research.22(2): 274–85.doi:10.1359/jbmr.061110.PMC4548943.PMID17129178.
- ^Azarpeykan S, Dittmer KE, Marshall JC, Perera KC, Gee EK, Acke E, Thompson KG (2016-09-15)."Evaluation and Comparison of Vitamin D Responsive Gene Expression in Ovine, Canine and Equine Kidney".PLOS ONE.11(9): e0162598.Bibcode:2016PLoSO..1162598A.doi:10.1371/journal.pone.0162598.PMC5025205.PMID27632366.
- ^Stulc J (July 1997). "Placental transfer of inorganic ions and water".Physiological Reviews.77(3): 805–36.doi:10.1152/physrev.1997.77.3.805.PMID9234966.
- ^Kovacs CS, Kronenberg HM (December 1997)."Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation".Endocrine Reviews.18(6): 832–72.doi:10.1210/edrv.18.6.0319.PMID9408745.
- ^Sharma D, Shastri S, Sharma P (January 2016)."Intrauterine Growth Restriction: Antenatal and Postnatal Aspects".Clinical Medicine Insights. Pediatrics.10:67–83.doi:10.4137/cmped.s40070.PMC4946587.PMID27441006.
- ^abBernucci L, Henríquez M, Díaz P, Riquelme G (November 2006). "Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane".Placenta.27(11–12): 1082–95.doi:10.1016/j.placenta.2005.12.007.hdl:10533/178038.PMID16564089.
- ^Lee GS, Jeung EB (July 2007). "Uterine TRPV6 expression during the estrous cycle and pregnancy in a mouse model".American Journal of Physiology. Endocrinology and Metabolism.293(1): E132-8.doi:10.1152/ajpendo.00666.2006.PMID17374692.
- ^Moreau R, Hamel A, Daoud G, Simoneau L, Lafond J (November 2002)."Expression of calcium channels along the differentiation of cultured trophoblast cells from human term placenta".Biology of Reproduction.67(5): 1473–9.doi:10.1095/biolreprod.102.005397.PMID12390878.S2CID23847059.
- ^Yang H, Kim TH, An BS, Choi KC, Lee HH, Kim JM, Jeung EB (March 2013). "Differential expression of calcium transport channels in placenta primary cells and tissues derived from preeclamptic placenta".Molecular and Cellular Endocrinology.367(1–2): 21–30.doi:10.1016/j.mce.2012.12.012.PMID23267838.S2CID5276054.
- ^Miura S, Sato K, Kato-Negishi M, Teshima T, Takeuchi S (November 2015)."Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6".Nature Communications.6(1): 8871.Bibcode:2015NatCo...6.8871M.doi:10.1038/ncomms9871.PMC4660203.PMID26563429.
- ^Ecroyd H, Asquith KL, Jones RC, Aitken RJ (April 2004)."The development of signal transduction pathways during epididymal maturation is calcium dependent".Developmental Biology.268(1): 53–63.doi:10.1016/j.ydbio.2003.12.015.PMID15031104.
- ^Gao D, Zhang BL, Leung MC, Au SC, Wong PY, Shum WW (August 2016)."Coupling of TRPV6 and TMEM16A in epithelial principal cells of the rat epididymis".The Journal of General Physiology.148(2): 161–82.doi:10.1085/jgp.201611626.PMC4969799.PMID27481714.
- ^abcdLieben L, Benn BS, Ajibade D, Stockmans I, Moermans K, Hediger MA, et al. (August 2010)."Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis".Bone.47(2): 301–8.doi:10.1016/j.bone.2010.04.595.PMC2902603.PMID20399919.
- ^abcLieben L, Carmeliet G (2012)."The Involvement of TRP Channels in Bone Homeostasis".Frontiers in Endocrinology.3:99.doi:10.3389/fendo.2012.00099.PMC3422722.PMID22934090.
- ^Tu CL, Bikle DD (June 2013)."Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function".Best Practice & Research. Clinical Endocrinology & Metabolism.27(3): 415–27.doi:10.1016/j.beem.2013.03.002.PMC3713412.PMID23856269.
- ^abcdNakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC (May 2007)."Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels".American Journal of Physiology. Renal Physiology.292(5): F1314-21.doi:10.1152/ajprenal.00432.2006.PMC2515270.PMID17200157.
- ^abDe Clercq K, Held K, Van Bree R, Meuleman C, Peeraer K, Tomassetti C, et al. (June 2015)."Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle".Human Reproduction.30(6): 1421–36.doi:10.1093/humrep/dev068.PMID25820697.
- ^abcYang H, Choi KC, Hyun SH, Jeung EB (April 2011). "Coexpression and estrogen-mediated regulation of TRPV6 and PMCA1 in the human endometrium during the menstrual cycle".Molecular Reproduction and Development.78(4): 274–82.doi:10.1002/mrd.21303.PMID21400627.S2CID21140465.
- ^abDe Clercq K, Van den Eynde C, Hennes A, Van Bree R, Voets T, Vriens J (March 2017)."The functional expression of transient receptor potential channels in the mouse endometrium".Human Reproduction.32(3): 615–630.doi:10.1093/humrep/dew344.PMID28077439.S2CID3409475.
- ^Suzuki Y, Chitayat D, Sawada H, Deardorff MA, McLaughlin HM, Begtrup A, Millar K, Harrington J, Chong K, Roifman M, Grand K, Tominaga M, Takada F, Shuster S, Obara M, Mutoh H, Kushima R, Nishimura G (June 2018)."TRPV6 Variants Interfere with Maternal-Fetal Calcium Transport through the Placenta and Cause Transient Neonatal Hyperparathyroidism".American Journal of Human Genetics.102(6): 1104–1114.doi:10.1016/j.ajhg.2018.04.006.PMC5992228.PMID29861107.
- ^Yamashita S, Mizumoto H, Sawada H, Suzuki Y, Hata D (March 2019)."TRPV6 Gene Mutation in a Dizygous Twin With Transient Neonatal Hyperparathyroidism".Journal of the Endocrine Society.3(3): 602–606.doi:10.1210/js.2018-00374.PMC6389352.PMID30820485.
- ^Burren CP, Caswell R, Castle B, Welch CR, Hilliard TN, Smithson SF, Ellard S (September 2018)."TRPV6 compound heterozygous variants result in impaired placental calcium transport and severe undermineralization and dysplasia of the fetal skeleton".American Journal of Medical Genetics. Part A.176(9): 1950–1955.doi:10.1002/ajmg.a.40484.PMC6563443.PMID30144375.
- ^abZou WB, Wang YC, Ren XL, Wang L, Deng SJ, Mao XT, et al. (August 2020)."TRPV6 variants confer susceptibility to chronic pancreatitis in the Chinese population".Human Mutation.41(8): 1351–1357.doi:10.1002/humu.24032.PMID32383311.
- ^abcdMasamune A, Kotani H, Sörgel FL, Chen JM, Hamada S, Sakaguchi R, et al. (May 2020)."Variants That Affect Function of Calcium Channel TRPV6 Are Associated With Early-Onset Chronic Pancreatitis".Gastroenterology.158(6): 1626–1641.e8.doi:10.1053/j.gastro.2020.01.005.PMID31930989.
- ^abSuzuki, Yoshiro Pasch, Andreas Bonny, Olivier Mohaupt, Markus G. Hediger, Matthias A. Frey, Felix J. (2017-08-02).Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation.Vol. 17. pp. 1613–8.doi:10.1093/hmg/ddn048.OCLC1156692319.PMID18276610.
{{cite book}}:|journal=ignored (help)CS1 maint: multiple names: authors list (link) - ^abWalters JR, Balesaria S, Chavele KM, Taylor V, Berry JL, Khair U, et al. (November 2006)."Calcium channel TRPV6 expression in human duodenum: different relationships to the vitamin D system and aging in men and women".Journal of Bone and Mineral Research.21(11): 1770–7.doi:10.1359/jbmr.060721.PMID17002582.S2CID22847166.
- ^abSherwood J, Bertrand J, Seidemann M, Dell'Accio F, Pap T (April 2016)."Activation of the transient receptor potential cation channel TRPC6 is required for chondrocyte phenotypic stability".Osteoarthritis and Cartilage.24:S152–S153.doi:10.1016/j.joca.2016.01.298.ISSN1063-4584.
- ^abcdDror AA, Brownstein Z, Avraham KB (2011)."Integration of human and mouse genetics reveals pendrin function in hearing and deafness".Cellular Physiology and Biochemistry.28(3): 535–44.doi:10.1159/000335163.PMC3709173.PMID22116368.
- ^Sun F, Xiao L, Jang XX, Xiong Y, Li Q, Yue XJ, et al. (October 2016). "TRPV6 is a prognostic marker in early-stage cervical squamous cell carcinoma".Tumour Biology.37(12): 15743–15751.doi:10.1007/s13277-016-5368-4.PMID27747588.S2CID6445506.
- ^abFan H, Shen YX, Yuan YF (2014-03-30)."Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection".Asian Pacific Journal of Cancer Prevention.15(6): 2559–63.doi:10.7314/apjcp.2014.15.6.2559.PMID24761864.
- ^Wu Y, Miyamoto T, Li K, Nakagomi H, Sawada N, Kira S, et al. (December 2011). "Decreased expression of the epithelial Ca2+ channel TRPV5 and TRPV6 in human renal cell carcinoma associated with vitamin D receptor".The Journal of Urology.186(6): 2419–25.doi:10.1016/j.juro.2011.07.086.PMID22019165.
- ^abcdePeng JB, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, et al. (April 2001). "CaT1 expression correlates with tumor grade in prostate cancer".Biochemical and Biophysical Research Communications.282(3): 729–34.doi:10.1006/bbrc.2001.4638.PMID11401523.
- ^abFixemer T, Wissenbach U, Flockerzi V, Bonkhoff H (October 2003)."Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression".Oncogene.22(49): 7858–61.doi:10.1038/sj.onc.1206895.PMID14586412.S2CID23626142.
- ^Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, et al. (2011)."High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters".Cellular Physiology and Biochemistry.28(5): 813–22.doi:10.1159/000335795.PMID22178934.S2CID45740662.
- ^abBolanz KA, Hediger MA, Landowski CP (February 2008)."The role of TRPV6 in breast carcinogenesis".Molecular Cancer Therapeutics.7(2): 271–9.doi:10.1158/1535-7163.mct-07-0478.PMID18245667.S2CID17158946.
- ^Peters AA, Simpson PT, Bassett JJ, Lee JM, Da Silva L, Reid LE, et al. (October 2012)."Calcium channel TRPV6 as a potential therapeutic target in estrogen receptor-negative breast cancer".Molecular Cancer Therapeutics.11(10): 2158–68.doi:10.1158/1535-7163.mct-11-0965.PMID22807578.S2CID207614323.
- ^abcLehen'kyi V, Flourakis M, Skryma R, Prevarskaya N (November 2007)."TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways".Oncogene.26(52): 7380–5.doi:10.1038/sj.onc.1210545.PMID17533368.S2CID482760.
- ^Lehen'kyi V, Raphaël M, Oulidi A, Flourakis M, Khalimonchyk S, Kondratskyi A, et al. (February 2011)."TRPV6 determines the effect of vitamin D3 on prostate cancer cell growth".PLOS ONE.6(2): e16856.Bibcode:2011PLoSO...616856L.doi:10.1371/journal.pone.0016856.PMC3037935.PMID21347289.
- ^Bolanz KA, Kovacs GG, Landowski CP, Hediger MA (December 2009)."Tamoxifen inhibits TRPV6 activity via estrogen receptor-independent pathways in TRPV6-expressing MCF-7 breast cancer cells".Molecular Cancer Research.7(12): 2000–10.doi:10.1158/1541-7786.mcr-09-0188.PMID19996302.S2CID26337876.
- ^Peleg S, Sellin JH, Wang Y, Freeman MR, Umar S (September 2010)."Suppression of aberrant transient receptor potential cation channel, subfamily V, member 6 expression in hyperproliferative colonic crypts by dietary calcium".American Journal of Physiology. Gastrointestinal and Liver Physiology.299(3): G593-601.doi:10.1152/ajpgi.00193.2010.PMC2950683.PMID20508153.
- ^Arbabian A, Iftinca M, Altier C, Singh PP, Isambert H, Coscoy S (December 2020)."Mutations in calmodulin-binding domains of TRPV4/6 channels confer invasive properties to colon adenocarcinoma cells".Channels.14(1): 101–109.doi:10.1080/19336950.2020.1740506.PMC7153789.PMID32186440.
- ^Ishizawa M, Akagi D, Yamamoto J, Makishima M (September 2017). "3 enhances TRPV6 transcription through p38 MAPK activation and GADD45 expression".The Journal of Steroid Biochemistry and Molecular Biology.172:55–61.doi:10.1016/j.jsbmb.2017.05.013.PMID28578001.S2CID206502344.
- ^Dai W, Bai Y, Hebda L, Zhong X, Liu J, Kao J, Duan C (April 2014)."Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell proliferation".Cell Death and Differentiation.21(4): 568–81.doi:10.1038/cdd.2013.177.PMC3950320.PMID24336047.
- ^Skrzypski M, Kołodziejski PA, Mergler S, Khajavi N, Nowak KW, Strowski MZ (August 2016)."TRPV6 modulates proliferation of human pancreatic neuroendocrine BON-1 tumour cells".Bioscience Reports.36(4).doi:10.1042/bsr20160106.PMC4995500.PMID27450545.
- ^Song H, Dong M, Zhou J, Sheng W, Li X, Gao W (March 2018)."Expression and prognostic significance of TRPV6 in the development and progression of pancreatic cancer".Oncology Reports.39(3): 1432–1440.doi:10.3892/or.2018.6216.PMID29344675.
- ^Chow J, Norng M, Zhang J, Chai J (April 2007)."TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells--Mechanisms behind a possible new" hot "cancer treatment".Biochimica et Biophysica Acta (BBA) - Molecular Cell Research.1773(4): 565–76.doi:10.1016/j.bbamcr.2007.01.001.PMID17292493.
- ^Zhang SS, Xie X, Wen J, Luo KJ, Liu QW, Yang H, et al. (January 2016)."TRPV6 plays a new role in predicting survival of patients with esophageal squamous cell carcinoma".Diagnostic Pathology.11(1): 14.doi:10.1186/s13000-016-0457-7.PMC4730645.PMID26818094.
- ^abcSong Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, et al. (September 2003)."Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice".Endocrinology.144(9): 3885–94.doi:10.1210/en.2003-0314.PMID12933662.
- ^abcdefBrown AJ, Krits I, Armbrecht HJ (May 2005). "Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels".Archives of Biochemistry and Biophysics.437(1): 51–8.doi:10.1016/j.abb.2005.02.007.PMID15820216.
- ^abMeyer MB, Watanuki M, Kim S, Shevde NK, Pike JW (June 2006)."The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells".Molecular Endocrinology.20(6): 1447–61.doi:10.1210/me.2006-0031.PMID16574738.
- ^abSong Y, Kato S, Fleet JC (February 2003)."Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA".The Journal of Nutrition.133(2): 374–80.doi:10.1093/jn/133.2.374.PMID12566470.
- ^Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G (January 2016)."Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects".Physiological Reviews.96(1): 365–408.doi:10.1152/physrev.00014.2015.PMC4839493.PMID26681795.
- ^Bouillon R, Van Cromphaut S, Carmeliet G (February 2003). "Intestinal calcium absorption: Molecular vitamin D mediated mechanisms".Journal of Cellular Biochemistry.88(2): 332–9.doi:10.1002/jcb.10360.PMID12520535.S2CID9853381.
- ^Replogle RA, Li Q, Wang L, Zhang M, Fleet JC (March 2014)."Gene-by-diet interactions influence calcium absorption and bone density in mice".Journal of Bone and Mineral Research.29(3): 657–65.doi:10.1002/jbmr.2065.PMC10591522.PMID23955923.S2CID1546230.
- ^abVan Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG, et al. (October 2003)."Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms".Journal of Bone and Mineral Research.18(10): 1725–36.doi:10.1359/jbmr.2003.18.10.1725.PMID14584880.S2CID25346500.
- ^Charoenphandhu N, Nakkrasae LI, Kraidith K, Teerapornpuntakit J, Thongchote K, Thongon N, Krishnamra N (September 2009). "Two-step stimulation of intestinal Ca(2+) absorption during lactation by long-term prolactin exposure and suckling-induced prolactin surge".American Journal of Physiology. Endocrinology and Metabolism.297(3): E609-19.doi:10.1152/ajpendo.00347.2009.PMID19567804.
- ^abBeggs MR, Lee JJ, Busch K, Raza A, Dimke H, Weissgerber P, et al. (2019)."v1.3 Mediate Distal Small Intestine Calcium Absorption Before Weaning".Cellular and Molecular Gastroenterology and Hepatology.8(4): 625–642.doi:10.1016/j.jcmgh.2019.07.005.PMC6889763.PMID31398491.
- ^van Abel M, Huybers S, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ (December 2006). "Age-dependent alterations in Ca2+ homeostasis: role of TRPV5 and TRPV6".American Journal of Physiology. Renal Physiology.291(6): F1177-83.doi:10.1152/ajprenal.00038.2006.PMID16705151.
- ^Nie X, Jin H, Wen G, Xu J, An J, Liu X, et al. (January 2020). "Estrogen Regulates Duodenal Calcium Absorption Through Differential Role of Estrogen Receptor on Calcium Transport Proteins".Digestive Diseases and Sciences.65(12): 3502–3513.doi:10.1007/s10620-020-06076-x.PMID31974908.S2CID210862678.
- ^Lee BM, Lee GS, Jung EM, Choi KC, Jeung EB (May 2009)."Uterine and placental expression of TRPV6 gene is regulated via progesterone receptor- or estrogen receptor-mediated pathways during pregnancy in rodents".Reproductive Biology and Endocrinology.7(1): 49.doi:10.1186/1477-7827-7-49.PMC2694200.PMID19457270.
- ^Park SY, Yoo YM, Jung EM, Jeung EB (April 2020). "The effect of steroid hormone on the expression of the calcium-processing proteins in the immature female rat brain".Journal of Chemical Neuroanatomy.105:101767.doi:10.1016/j.jchemneu.2020.101767.PMID32061997.S2CID211105316.
- ^abKim MH, Lee GS, Jung EM, Choi KC, Jeung EB (July 2009). "The negative effect of dexamethasone on calcium-processing gene expressions is associated with a glucocorticoid-induced calcium-absorbing disorder".Life Sciences.85(3–4): 146–52.doi:10.1016/j.lfs.2009.05.013.PMID19490920.
- ^abKoo TH, Yang H, Jeung EB (2011-07-01). "Expression of Calcium Transport Genes in the Placenta of Calbindin-D9k and -D28k Knockout Mice".Biology of Reproduction.85(Suppl_1): 449.doi:10.1093/biolreprod/85.s1.449.ISSN0006-3363.
- ^abSopjani M, Kunert A, Czarkowski K, Klaus F, Laufer J, Föller M, Lang F (February 2010). "Regulation of the Ca(2+) channel TRPV6 by the kinases SGK1, PKB/Akt, and PIKfyve".The Journal of Membrane Biology.233(1–3): 35–41.doi:10.1007/s00232-009-9222-0.PMID20041238.S2CID25349984.
Further reading[edit]
- Heiner I, Eisfeld J, Lückhoff A (2004). "Role and regulation of TRP channels in neutrophil granulocytes".Cell Calcium.33(5–6): 533–40.doi:10.1016/S0143-4160(03)00058-7.PMID12765698.
- Clapham DE, Julius D, Montell C, Schultz G (December 2005). "International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels".Pharmacological Reviews.57(4): 427–50.doi:10.1124/pr.57.4.6.PMID16382100.S2CID17936350.
- Wissenbach U, Niemeyer BA (2007). "TRPV6".Transient Receptor Potential (TRP) Channels.Handbook of Experimental Pharmacology. Vol. 179. pp. 221–34.doi:10.1007/978-3-540-34891-7_13.ISBN978-3-540-34889-4.PMID17217060.
- Schoeber JP, Hoenderop JG, Bindels RJ (February 2007). "Concerted action of associated proteins in the regulation of TRPV5 and TRPV6".Biochemical Society Transactions.35(Pt 1): 115–9.doi:10.1042/BST0350115.PMID17233615.
External links[edit]
- TRPV+Cation+Channelsat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- TRPV6+protein,+humanat the U.S. National Library of MedicineMedical Subject Headings(MeSH)





