Tebbe's reagent
| |

| |
| Names | |
|---|---|
| IUPAC name
μ-Chloro[di(cyclopenta-2,4-dien-1-yl)]dimethyl(μ-methylene)titaniumaluminum
| |
| Other names
Tebbe reagent
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.162 |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C13H18AlClTi | |
| Molar mass | 284.60 g/mol |
| Solubilityin other solvents | toluene, benzene, dichloromethane, THF (low temperatures only) |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
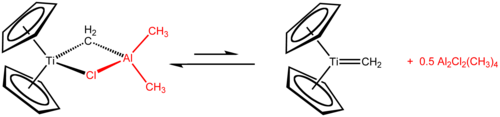
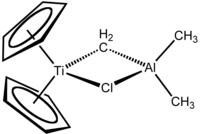
Tebbe's reagentis theorganometallic compoundwith the formula (C5H5)2TiCH2ClAl(CH3)2.It is used in themethylidenationofcarbonylcompounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2derivative.[1]It is a red solid that ispyrophoricin the air, and thus is typically handled withair-free techniques.It was originally synthesized byFred TebbeatDuPont Central Research.
Tebbe's reagent contains twotetrahedralmetal centers linked by a pair ofbridging ligands.The titanium has twocyclopentadienyl([C
5H
5]−
,or Cp) rings and aluminium has two methyl groups. The titanium and aluminium atoms are linked together by both amethylene bridge(-CH2-) and a chloride atom in a nearlysquare-planar(Ti–CH2–Al–Cl) geometry.[2]The Tebbe reagent was the first reported compound where a methylene bridge connects a transition metal (Ti) and a main group metal (Al).[3]
Preparation
[edit]The Tebbe reagent is synthesized fromtitanocene dichlorideandtrimethylaluminiumintoluenesolution.[3][4]
- Cp2TiCl2+ 2 Al(CH3)3→ CH4+ Cp2TiCH2AlCl(CH3)2+ Al(CH3)2Cl
After about 3 days, the product is obtained after recrystallization to remove Al(CH3)2Cl.[3]Although syntheses using the isolated Tebbe reagent give a cleaner product, successful procedures using the reagent "in situ" have been reported.[5][6]Instead of isolating the Tebbe reagent, the solution is merely cooled in an ice bath or dry ice bath before adding the starting material.
An alternative but less convenient synthesis entails the use ofdimethyltitanocene(Petasis reagent):[7]
- Cp2Ti(CH3)2+ Al(CH3)2Cl → Cp2TiCH2AlCl(CH3)2+ CH4
One drawback to this method, aside from requiring Cp2Ti(CH3)2,is the difficulty of separating product from unreacted starting reagent.
Reaction mechanism
[edit]Tebbe's reagent itself does not react with carbonyl compounds, but must first be treated with a mildLewis base,such aspyridine,which generates the activeSchrock carbene.
Also analogous to the Wittig reagent, the reactivity appears to be driven by the highoxophilicityof Ti(IV). The Schrock carbene (1) reacts with carbonyl compounds (2) to give a postulated oxatitanacyclobutane intermediate (3). This cyclic intermediate has never been directly isolated, presumably because it breaks down immediately to the produce the desiredalkene(5).
Scope
[edit]The Tebbe reagent is used inorganic synthesisfor carbonyl methylidenation.[8] [9][10]This conversion can also be effected using theWittig reaction,although the Tebbe reagent is more efficient especially for sterically encumbered carbonyls. Furthermore, the Tebbe reagent is less basic than the Wittig reagent and does not give the β-elimination products.
Methylidenation reactions also occur foraldehydesas well asesters,lactonesandamides.The Tebbe reagent converts esters and lactones to enol ethers and amides to enamines. In compounds containing both ketone and ester groups, the ketone selectively reacts in the presence of one equivalent of the Tebbe reagent.
The Tebbe reagent methylidenates carbonyls without racemizing achiralα carbon. For this reason, the Tebbe reagent has found applications in reactions of sugars where maintenance ofstereochemistrycan be critical.[11]
The Tebbe reagent reacts withacid chloridesto form titanium enolates by replacing Cl−.
Modifications
[edit]It is possible to modify Tebbe's reagent through the use of different ligands. This can alter the reactivity of the complex, allowing for a broader range of reactions. For example,cyclopropanationcan be achieved using a chlorinated analogue.[12]
See also
[edit]Related organotitanium reagents and reactions
[edit]- Kulinkovich reaction
- Petasis reagent
- Lombardo reagent[13]
- McMurry reaction
Related methylidenation reactions
[edit]References
[edit]- ^F. N. Tebbe, G. W. Parshall and G. S. Reddy (1978). "Olefin homologation with titanium methylene compounds".J. Am. Chem. Soc.100(11): 3611–3613.doi:10.1021/ja00479a061.
- ^Thompson, Rick; Nakamaru-Ogiso, Eiko; Chen, Chun-Hsing; Pink, Maren; Mindiola, Daniel J. (2014). "Structural Elucidation of the Illustrious Tebbe Reagent".Organometallics.33(1): 429–432.doi:10.1021/om401108b.
- ^abcHerrmann, W.A., "The Methylene Bridge"Advances in Organometallic Chemistry1982,20,195–197.
- ^Straus, D. A., "μ-Chlorobis(cyclopentadienyl)(dimethylaluminium)-μ-methylenetitanium":Encyclopedia of Reagents for Organic Synthesis.John Wiley, London, 2000.
- ^Pine, S. H.; Kim, V.; Lee, V. (1990). "Enol ethers by methylenation of esters: 1-Phenoxy-1-phenylethene and 3,4-dihydro-2-methylene-2H-1-benzopyran ".Org. Synth.69:72.doi:10.15227/orgsyn.069.0072.
- ^L. F. Cannizzo & R. H. Grubbs (1985). "In situ preparation of (μ-chloro)(μ-methylene)bis(cyclopentadienyl)(dimethylaluminum)titanium (Tebbe's reagent)".J. Org. Chem.50(13): 2386–2387.doi:10.1021/jo00213a040.
- ^Payack, J. F.; Hughes, D. L.; Cai, D.; Cottrell, I. F.; Verhoeven, T. R. (2004)."Dimethyltitanocene".Organic Syntheses
{{cite journal}}:CS1 maint: multiple names: authors list (link);Collected Volumes,vol. 10, p. 355. - ^Hartley, Richard C.; Li, Jianfeng; Main, Calver A.; McKiernan, Gordon J. (2007). "Titanium carbenoid reagents for converting carbonyl groups into alkenes".Tetrahedron.63(23): 4825–4864.doi:10.1016/j.tet.2007.03.015.
- ^Pine, S. H.Org. React.1993,43,1. (Review)
- ^Beadham, I.; Micklefield, J.Curr. Org. Synth.2005,2,231–250. (Review)
- ^A. Marra, J. Esnault, A. Veyrieres and P. Sinay (1992). "Isopropenyl glycosides and congeners as novel classes of glycosyl donors: theme and variations".J. Am. Chem. Soc.114(16): 6354–6360.doi:10.1021/ja00042a010.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Unusual Ambiphilic Carbenoid Equivalent in Amide CyclopropanationKuo-Wei Lin, Shiuan Yan, I-Lin Hsieh, and Tu-Hsin YanOrg. Lett.;2006;8(11) pp 2265 – 2267;Abstract
- ^Luciano Lombardo (1987). "Methylenation of Carbonyl Compounds: (+)-3-Methylene-cis-p-menthane ".Organic Syntheses.65:81.doi:10.15227/orgsyn.065.0081..