Telophase
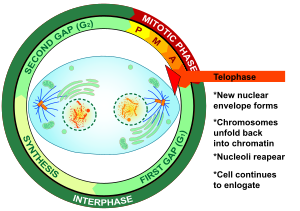
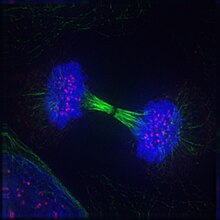
Telophase(fromAncient Greekτέλος(télos)'end, result, completion' andφάσις(phásis)'appearance') is the final stage in bothmeiosisandmitosisin aeukaryoticcell.During telophase, the effects ofprophaseandprometaphase(thenucleolusand nuclear membrane disintegrating) are reversed. Aschromosomesreach the cell poles, anuclear envelopeis re-assembled around each set ofchromatids,thenucleolireappear, and chromosomes begin to decondense back into the expandedchromatinthat is present duringinterphase.Themitotic spindleis disassembled and remaining spindlemicrotubulesare depolymerized. Telophase accounts for approximately 2% of thecell cycle's duration.
Cytokinesistypically begins before late telophase[1]and, when complete, segregates the two daughternucleibetween a pair of separate daughter cells.
Telophase is primarily driven by thedephosphorylationof mitoticcyclin-dependent kinase(Cdk) substrates.[2]
Dephosphorylation of Cdk substrates
[edit]Thephosphorylationof the protein targets of M-Cdks (Mitotic Cyclin-dependent Kinases) drives spindle assembly, chromosome condensation and nuclear envelope breakdown in early mitosis. The dephosphorylation of these same substrates drives spindle disassembly, chromosome decondensation and the reformation of daughter nuclei in telophase. Establishing a degree of dephosphorylation permissive to telophase events requires both the inactivation of Cdks and the activation ofphosphatases.[citation needed]
Cdk inactivation is primarily the result of the destruction of its associatedcyclin.Cyclins are targeted forproteolytic degradationby theanaphase promoting complex(APC), also known as the cyclosome,[3]a ubiquitin-ligase. The active,CDC20-bound APC (APC/CCDC20) targets mitotic cyclins for degradation starting inanaphase.[4]Experimental addition of non-degradable M-cyclin to cells induces cell cycle arrest in a post-anaphase/pre-telophase-like state with condensed chromosomes segregated to cell poles, an intact mitotic spindle, and no reformation of the nuclear envelope. This has been shown in frog (Xenopus)eggs, fruit flies (Drosophilla melanogaster), budding (Saccharomyces cerevisiae) and fission (Schizosaccharomyces pombe) yeast, and in multiple human cell lines.[5]
The requirement for phosphatase activation can be seen in budding yeast, which do not have redundant phosphatases for mitotic exit and rely on the phosphatasecdc14.Blocking cdc14 activation in these cells results in the same phenotypic arrest as does blocking M-cyclin degradation.[4][2]
Historically, it has been thought thatanaphaseand telophase are events that occur passively after satisfaction of thespindle-assembly checkpoint(SAC) that defines themetaphase-anaphase transition.[6]However, the existence of differential phases to cdc14 activity between anaphase and telophase is suggestive of additional, unexplored late-mitotic checkpoints.Cdc14 is activated by its release into the nucleus, from sequestration in the nucleolus, and subsequent export into the cytoplasm. The Cdc-14 Early Anaphase Release pathway, which stabilizes the spindle, also releases cdc14 from the nucleolus but restricts it to the nucleus. Complete release and maintained activation of cdc14 is achieved by the separate Mitotic Exit Network (MEN) pathway to a sufficient degree (to trigger the spindle disassembly and nuclear envelope assembly) only after late anaphase.[7][8]
Cdc14-mediated dephosphorylation activates downstream regulatory processes unique to telophase. For example, the dephosphorylation ofCDH1allows the APC/C to bind CDH1. APC/CCDH1targets CDC20 for proteolysis, resulting in a cellular switch from APC/CCDC20to APC/CCDH1activity.[5]The ubiquitination of mitotic cyclins continues along with that of APC/CCDH1-specific targets such as the yeast mitotic spindle component, Ase1,[2]and cdc5, the degradation of which is required for the return of cells to theG1 phase.[7]
Additional mechanisms driving telophase
[edit]A shift in the whole-cellphosphoproteinprofile is only the broadest of many regulatory mechanisms contributing to the onset of individual telophase events.
- The anaphase-mediated distancing of chromosomes from the metaphase plate may trigger spatial cues for the onset of telophase.[6]
- An important regulator and effector of telophase iscdc48(homologous to yeast cdc48 is humanp97,both structurally and functionally), a protein that mechanically employs itsATPaseactivity to alter target protein conformation. Cdc48 is necessary for spindle disassembly, nuclear envelope assembly, and chromosome decondensation. Cdc48 modifies proteins structurally involved in these processes and also some ubiquitinated proteins which are thus targeted to theproteasome.[2][9][10]
Mitotic spindle disassembly
[edit]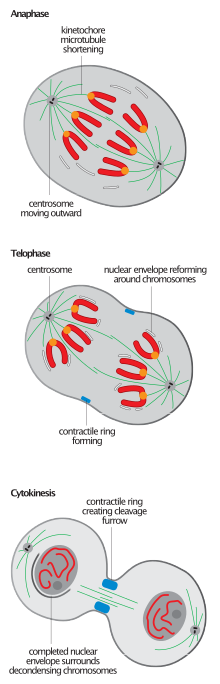
The breaking of the mitotic spindle, common to the completion of mitosis in all eukaryotes, is the event most often used to define the anaphase-B to telophase transition,[2][6]although the initiation of nuclear reassembly tends to precede that of spindle disassembly.[11]
Spindle disassembly is an irreversible process which must effect not the ultimate degradation, but the reorganization of constituent microtubules; microtubules are detached fromkinetochoresandspindle pole bodiesand return to their interphase states.[citation needed]
Spindle depolymerization during telophase occurs from the plus end and is, in this way, a reversal of spindle assembly.[12]Subsequent microtubule array assembly is, unlike that of the polarized spindle, interpolar. This is especially apparent in animal cells which must immediately, following mitotic spindle disassembly, establish the antiparallel bundle of microtubules known as thecentral spindlein order to regulate cytokinesis.[2]The ATPase p97 is required for the establishment of the relatively stable and long interphasemicrotubule arraysfollowing disassembly of the highly dynamic and relatively short mitotic ones.[9]
While spindle assembly has been well studied and characterized as a process where tentative structures are edified by the SAC, the molecular basis of spindle disassembly is not understood in comparable detail. The late-mitoticdephosphorylation cascadeof M-Cdk substrates by the MEN is broadly held to be responsible for spindle disassembly. The phosphorylation states of microtubule stabilizing and destabilizing factors, as well as microtubule nucleators are key regulators of their activities.[9]For example, NuMA is a minus-end crosslinking protein and Cdk substrate whose dissociation from the microtubule is effected by its dephosphorylation during telophase.[2]
A general model for spindle disassembly in yeast is that the three functionally overlapping subprocesses of spindle disengagement, destabilization, and depolymerization are primarily effected by APC/CCDH1,microtubule-stabilizer-specific kinases, and plus-end directed microtubule depolymerases, respectively. These effectors are known to be highly conserved between yeast and higher eukaryotes. The APC/CCDH1targets crosslinking microtubule-associated proteins (NuMA, Ase1, Cin1 and more).AuroraB(yeast IpI1) phosphorylates the spindle-associated stabilizing proteinEB1(yeast Bim1), which then dissociates from microtubules, and the destabilizer She1, which then associates with microtubules.Kinesin8(yeast Kip3), an ATP-dependent depolymerase, accelerate microtubule depolymerization at the plus end. It was shown the concurrent disruption of these mechanisms, but not of any one, results in dramatic spindle hyperstability during telophase, suggesting functional overlap despite the diversity of the mechanisms.[13]
Nuclear envelope reassembly
[edit]The main components of the nuclear envelope are a double membrane,nuclear pore complexes,and anuclear laminainternal to the inner nuclear membrane. These components are dismantled during prophase and prometaphase and reconstructed during telophase, when the nuclear envelope reforms on the surface of separated sister chromatids.[14][15]The nuclear membrane is fragmented and partly absorbed by theendoplasmic reticulumduring prometaphase and the targeting of inner nuclear membrane protein-containing ERvesiclesto the chromatin occurs during telophase in a reversal of this process. Membrane-forming vesicles aggregate directly to the surface of chromatin, where theyfuselaterally into a continuous membrane.[2]
Ran-GTPis required for early nuclear envelope assembly at the surface of the chromosomes: it releases envelope components sequestered byimportin βduring early mitosis. Ran-GTP localizes near chromosomes throughout mitosis, but does not trigger the dissociation of nuclear envelope proteins from importin β until M-Cdk targets are dephosphorylated in telophase.[2]These envelope components include several nuclear pore components, the most studied of which is the nuclear pore scaffold proteinELYS,which can recognize DNA regions rich in A:T base pairs (in vitro), and may therefore bind directly to the DNA.[16]However, experiments inXenopusegg extracts have concluded that ELYS fails to associate with bare DNA and will only directly bindhistonedimers and nucleosomes.[17]After binding to chromatin, ELYS recruits other components of the nuclear pore scaffold and nuclear pore trans-membrane proteins. The nuclear pore complex is assembled and integrated in the nuclear envelope in an organized manner, consecutively adding Nup107-160,POM121,and FG Nups.[18]
It is debated whether the mechanism of nuclear membrane reassembly involves initial nuclear pore assembly and subsequent recruitment of membrane vesicles around the pores or if the nuclear envelope forms primarily from extended ER cisternae, preceding nuclear pore assembly:
- In cells where the nuclear membrane fragments into non-ER vesicles during mitosis, a Ran-GTP–dependent pathway can direct these discrete vesicle populations to chromatin where they fuse to reform the nuclear envelope.[19][16]
- In cells where the nuclear membrane is absorbed into the endoplasmic reticulum during mitosis, reassembly involves the lateral expansion around the chromatin with stabilization of the expanding membrane over the surface of the chromatin.[20]Studies claiming this mechanism is a prerequisite to nuclear pore formation have found that bare-chromatin-associated Nup107–160 complexes are present in single units instead of as assembled pre-pores.[21][16]
The envelope smoothens and expands following its enclosure of the whole chromatid set. This probably occurs due to the nuclear pores' import oflamin,which can be retained within a continuous membrane. The nuclear envelopes ofXenopusegg extracts failed to smoothen when nuclear import of lamin was inhibited, remaining wrinkled and closely bound to condensed chromosomes.[22]However, in the case of ER lateral expansion, nuclear import is initiated before completion of the nuclear envelope reassembly, leading to a temporary intra-nuclear protein gradient between the distal and medial aspects of the forming nucleus.[18]
Lamin subunits disassembled in prophase are inactivated and sequestered during mitosis. Lamina reassembly is triggered by lamin dephosphorylation (and additionally by methyl-esterificationofCOOHresidues onlamin-B). Lamin-B can target chromatin as early as mid-anaphase. During telophase, when nuclear import is reestablished,lamin-Aenters the reforming nucleus but continues to slowly assemble into the peripheral lamina over several hours in throughout the G1 phase.[16]
Xenopusegg extracts and human cancer cell lines have been the primary models used for studying nuclear envelope reassembly.[18]
Yeast lack lamins; their nuclear envelope remains intact throughout mitosis and nuclear division happens during cytokinesis.[23][11]
Chromosome decondensation
[edit]Chromosome decondensation (also known as relaxation or decompaction) into expanded chromatin is necessary for the cell's resumption of interphase processes, and occurs in parallel to nuclear envelope assembly during telophase in many eukaryotes.[2]MEN-mediated Cdk dephosphorylation is necessary for chromosome decondensation.[2][5]
In vertebrates, chromosome decondensation is initiated only afternuclear importis reestablished. If lamin transport through nuclear pores is prevented, chromosomes remain condensed following cytokinesis, and cells fail to reenter the next S phase.[16]In mammals, DNA licensing for S phase (the association of chromatin to the multiple protein factors necessary for its replication) also occurs coincidentally with the maturation of the nuclear envelope during late telophase.[24][25]This can be attributed to and provides evidence for the nuclear import machinery's reestablishment of interphase nuclear and cytoplasmic protein localizations during telophase.
See also
[edit]- Cytoskeleton– Network of filamentous proteins that forms the internal framework of cells
References
[edit]- ^Reece, Jane; Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Jackson, Robert (2011). Campbell Biology (10th ed.). Pearson.ISBN978-0-321-77565-8.
- ^abcdefghijkMorgan D (2007).The Cell Cycle.London, UK: New Science Press Ltd. pp. 154–155.ISBN978-0-9539181-2-6.
- ^Juang YL, Huang J, Peters JM, McLaughlin ME, Tai CY, Pellman D (February 1997). "APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle".Science.275(5304): 1311–4.doi:10.1126/science.275.5304.1311.PMID9036857.S2CID12265554.
- ^abAlberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2015).Molecular Biology of the Cell(6th ed.). New York, NY: Garland Science, Taylor and Francis Group. pp. 995–996.ISBN978-0-8153-4432-2.
- ^abcInzé D (2007).Cell Cycle Control and Plant Development.Oxford, UK: Blackwell Publishing Ltd. pp.99–103.ISBN978-1-4051-5043-9.
- ^abcAfonso O, Matos I, Maiato H (2014)."Spatial control of the anaphase-telophase transition".Cell Cycle.13(19): 2985–6.doi:10.4161/15384101.2014.959853.PMC4614036.PMID25486554.
- ^abMonje-Casas F, Queralt E (2017).The Mitotic Exit Network.New York, NY: Humana Press. pp. 3–8.ISBN9781493965007.
- ^Yellman CM, Roeder GS (2015)."Cdc14 Early Anaphase Release, FEAR, Is Limited to the Nucleus and Dispensable for Efficient Mitotic Exit".PLOS ONE.10(6): e0128604.Bibcode:2015PLoSO..1028604Y.doi:10.1371/journal.pone.0128604.PMC4474866.PMID26090959.
- ^abcCao K, Nakajima R, Meyer HH, Zheng Y (October 2003)."The AAA-ATPase Cdc48/p97 regulates spindle disassembly at the end of mitosis".Cell.115(3): 355–67.doi:10.1016/S0092-8674(03)00815-8.PMID14636562.
- ^Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW (December 2001). "Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly".Nature Cell Biology.3(12): 1086–91.doi:10.1038/ncb1201-1086.PMID11781570.S2CID19261807.
- ^abAist JR (2002-01-01). "Mitosis and motor proteins in the filamentous ascomycete, Nectria haematococca, and some related fungi".International Review of Cytology.212:239–63.doi:10.1016/S0074-7696(01)12007-3.ISBN9780123646163.PMID11804038.
- ^Woodruff JB (2011).Mechanisms of Mitotic Spindle Disassembly and Positioning in Saccharomyces cerevisiae(Thesis). UC Berkeley.
- ^Woodruff JB, Drubin DG, Barnes G (November 2010)."Mitotic spindle disassembly occurs via distinct subprocesses driven by the anaphase-promoting complex, Aurora B kinase, and kinesin-8".The Journal of Cell Biology.191(4): 795–808.doi:10.1083/jcb.201006028.PMC2983061.PMID21079246.
- ^Yael A, Choi J, DeSaix J, Jurukovski V, Wisem R, Rye C (2013).Biology.Rice University, Houston, Texas 77005: OpenStax College. pp. 281–283.ISBN978-1-938168-09-3.
{{cite book}}:CS1 maint: location (link) - ^Lodish, Harvey; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James (2000).Molecular Cell Biology. 4th edition.W H Freeman. pp. Section 13.4.
- ^abcdePollard TD, Earnshaw WC, Lippincott-Schwartz J, Johnson GT (2017).Cell Biology(3rd ed.). Philadelphia, PA: Elsevier. pp. 770–771.ISBN978-0-323-34126-4.
- ^Zierhut C, Jenness C, Kimura H, Funabiki H (July 2014)."Nucleosomal regulation of chromatin composition and nuclear assembly revealed by histone depletion".Nature Structural & Molecular Biology.21(7): 617–25.doi:10.1038/nsmb.2845.PMC4082469.PMID24952593.
- ^abcGay S, Foiani M (2015-01-01). "Nuclear envelope and chromatin, lock and key of genome integrity".International Review of Cell and Molecular Biology.317:267–330.doi:10.1016/bs.ircmb.2015.03.001.ISBN9780128022801.PMID26008788.
- ^Clarke PR, Zhang C (2004). "Spatial and temporal control of nuclear envelope assembly by Ran GTPase".Symposia of the Society for Experimental Biology(56): 193–204.PMID15565882.
- ^Hetzer MW (March 2010)."The nuclear envelope".Cold Spring Harbor Perspectives in Biology.2(3): a000539.doi:10.1101/cshperspect.a000539.PMC2829960.PMID20300205.
- ^Lu L, Ladinsky MS, Kirchhausen T (August 2011)."Formation of the postmitotic nuclear envelope from extended ER cisternae precedes nuclear pore assembly".The Journal of Cell Biology.194(3): 425–40.doi:10.1083/jcb.201012063.PMC3153650.PMID21825076.
- ^Wiese C, Goldberg MW, Allen TD, Wilson KL (July 1997). "Nuclear envelope assembly in Xenopus extracts visualized by scanning EM reveals a transport-dependent 'envelope smoothing' event".Journal of Cell Science.110(13): 1489–502.doi:10.1242/jcs.110.13.1489.PMID9224766.
- ^Taddei A, Schober H, Gasser SM (August 2010)."The budding yeast nucleus".Cold Spring Harbor Perspectives in Biology.2(8): a000612.doi:10.1101/cshperspect.a000612.PMC2908769.PMID20554704.
- ^Dimitrova DS, Prokhorova TA, Blow JJ, Todorov IT, Gilbert DM (January 2002)."Mammalian nuclei become licensed for DNA replication during late telophase".Journal of Cell Science.115(Pt 1): 51–9.doi:10.1242/jcs.115.1.51.PMC1255924.PMID11801723.
- ^Fukushima K, Wang M, Naito Y, Uchihashi T, Kato Y, Mukai S, Yabuta N, Nojima H (March 2017)."GAK is phosphorylated by c-Src and translocated from the centrosome to chromatin at the end of telophase".Cell Cycle.16(5): 415–427.doi:10.1080/15384101.2016.1241916.PMC5351929.PMID28135906.
External links
[edit] Media related toTelophaseat Wikimedia Commons
Media related toTelophaseat Wikimedia Commons
