Thiazole
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Thiazole | |||
| Other names
Thiazole
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.475 | ||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H3NS | |||
| Molar mass | 85.12g·mol−1 | ||
| Boiling point | 116 to 118 °C (241 to 244 °F; 389 to 391 K) | ||
| Acidity(pKa) | 2.5 (of conjugate acid)[1] | ||
| -50.55·10−6cm3/mol | |||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Thiazole(/ˈθaɪ.əzoʊl/), or1,3-thiazole,is a 5-memberedheterocyclic compoundthat contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with apyridine-like odor and the molecular formula C3H3NS.[2]The thiazole ring is notable as a component of thevitaminthiamine(B1).
Molecular and electronic structure
[edit]Thiazoles are members of theazoles,heterocycles that includeimidazolesandoxazoles.Thiazole can also be considered afunctional groupwhen part of a larger molecule.
Being planar thiazoles are characterized by significant pi-electrondelocalizationand have some degree ofaromaticity,more so than the correspondingoxazoles.This aromaticity is evidenced by the1H NMRchemical shift of the ring protons, which absorb between 7.27 and 8.77 ppm, indicating a strongdiamagnetic ring current.The calculated pi-electron density marks C5 as the primary site for electrophilic substitution, and C2-H as susceptible to deprotonation.
Occurrence of thiazoles and thiazolium salts
[edit]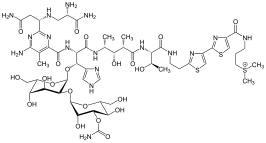
Thiazoles are found in a variety of specialized products, often fused with benzene derivatives, the so-called benzothiazoles. In addition to vitamin B1,the thiazole ring is found inepothilone.Other important thiazole derivatives arebenzothiazoles,for example, the firefly chemicalluciferin.Whereas thiazoles are well represented inbiomolecules,oxazoles are not. It is found in naturally occurring peptides, and utilised in the development of peptidomimetics (i.e. molecules that mimic the function and structure of peptides).[3]
Commercial significant thiazoles include mainly dyes andfungicides.Thifluzamide, Tricyclazole, andThiabendazoleare marketed for control of various agricultural pests. Another widely used thiazole derivative is the non-steroidal anti-inflammatory drugMeloxicam.The followinganthroquinonedyes contain benzothiazole subunits: Algol Yellow 8 (CAS# [6451-12-3]), Algol Yellow GC (CAS# [129-09-9]), Indanthren Rubine B (CAS# [6371-49-9]), Indanthren Blue CLG (CAS# [6371-50-2], and Indanthren Blue CLB (CAS#[6492-78-0]). These thiazole dye are used for dyeingcotton.
Synthesis
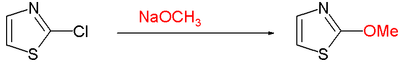
[edit]Various laboratory methods exist for theorganic synthesisof thiazoles. Prominent is the Hantzsch thiazole synthesis, which is a reaction betweenhaloketonesandthioamides.For example, 2,4-dimethylthiazole is synthesized fromthioacetamideandchloroacetone.[4]In theCook-Heilbron synthesis,thiazoles arise by the condensation of α-aminonitrile withcarbon disulfide.Thiazoles can be accessed by acylation of 2-aminothiolates, often available by theHerz reaction.
Biosynthesis
[edit]Thiazoles are generally formed via reactions ofcysteine,which provides the N-C-C-S backbone of the ring. Thiamine does not fit this pattern however. Several biosynthesis routes lead to the thiazole ring as required for the formation of thiamine.[5]Sulfur of the thiazole is derived from cysteine. In anaerobic bacteria, the CN group is derived from dehydroglycine.
Reactions
[edit]With a pKaof 2.5 for the conjugate acid, thiazoles are far less basic thanimidazole(pKa=7).[6]
Deprotonationwith strong bases occurs at C2-H. The negative charge on this position is stabilized as anylide.Hauser basesandorganolithium compoundsreact at this site, replacing the proton. 2-Lithiothiazoles are also generated by metal-halogen exchange from 2-bromothiazole.[7]
Electrophilic aromatic substitutionat C5 but requireactivating groupssuch as amethylgroup, as illustrated inbromination:
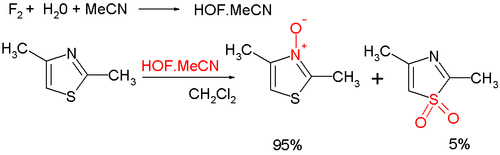
Oxidation at nitrogengives the aromatic thiazoleN-oxide;many oxidizing agents exist, such asmCPBA;a novel one ishypofluorous acidprepared fromfluorineand water inacetonitrile;some of the oxidation takes place at sulfur, leading to non-aromaticsulfoxide/sulfone:[8]ThiazoleN-oxides are useful in Palladium-catalysed C-H arylations, where theN-oxide is able to shift the reactivity to reliably favor the 2-position, and allows for these reactions to be carried out under much more mild conditions.[9]
- Thiazoles areformylsynthons;conversion ofR-thiato theR-CHOaldehyde takes place with,[7]respectively,methyl iodide(N-methylation),organic reductionwithsodium borohydride,andhydrolysiswithMercury(II) chloridein water.
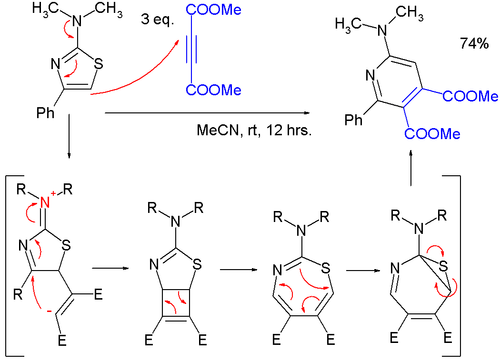
- Thiazoles can react incycloadditions,but in general at high temperatures due to favorable aromatic stabilization of the reactant;Diels-Alder reactionswithalkynesare followed by extrusion of sulfur, and the endproduct is apyridine;in one study,[10]a very mild reaction of a2-(dimethylamino)thiazolewithdimethyl acetylenedicarboxylate(DMAD) to a pyridine was found to proceed through azwitterionicintermediate in a formal [2+2]cycloaddition to a cyclobutene, then to a1,3-thiazepinein a 4-electronelectrocyclic ring openingand then to a7-thia-2-azanorcaradienein a 6-electron electrocyclic ring, closing before extruding the sulfur atom.
Thiazolium salts
[edit]Alkylationof thiazoles at nitrogen forms athiazoliumcation. Thiazolium salts are catalysts in theStetter reactionand theBenzoin condensation.Deprotonation ofN-alkyl thiazolium salts give thefree carbenes[11]andtransition metal carbene complexes.
Alagebriumis a thiazolium-based drug.
References
[edit]- ^Zoltewicz, J. A.; Deady, L. W. (1978). "Quaternization of Heteroaromatic Compounds: Quantitative Aspects".Advances in Heterocyclic Chemistry Volume 22.Vol. 22. pp. 71–121.doi:10.1016/S0065-2725(08)60103-8.ISBN9780120206223.
- ^Eicher, T.; Hauptmann, S. (2003).The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications.Wiley.ISBN978-3-527-30720-3.
- ^Mak, Jeffrey Y. W.; Xu, Weijun; Fairlie, David P. (2015-01-01).Peptidomimetics I(PDF).Topics in Heterocyclic Chemistry. Vol. 48. Springer Berlin Heidelberg. pp. 235–266.doi:10.1007/7081_2015_176.ISBN978-3-319-49117-2.
- ^George Schwarz (1945). "2,4-Dimethylthiazole".Organic Syntheses.25:35.doi:10.15227/orgsyn.025.0035.
- ^Kriek, M.; Martins, F.; Leonardi, R.; Fairhurst, S. A.; Lowe, D. J.; Roach, P. L. (2007)."Thiazole Synthase fromEscherichia coli:An Investigation of the Substrates and Purified Proteins Required for Activityin vitro"(PDF).J. Biol. Chem.282(24): 17413–17423.doi:10.1074/jbc.M700782200.PMID17403671.
- ^Thomas L. Gilchrist (1997).Heterocyclic Chemistry(3 ed.). Essex, England: Addison Wesley. p. 414.ISBN0-582-27843-0.
- ^abDondoni, A.; Merino, P. (1995). "Diastereoselective Homologation of D-(R)-Glyceraldehyde Acetonide using 2-(Trimethylsilyl)thiazole".72:21.doi:10.15227/orgsyn.072.0021.
{{cite journal}}:Cite journal requires|journal=(help) - ^Amir, E.; Rozen, S. (2006). "Easy Access to the Family of ThiazoleN-oxides using HOF·CH3CN ".Chemical Communications.2006(21): 2262–2264.doi:10.1039/b602594c.PMID16718323.
- ^Campeau, Louis-Charles; Bertrand-Laperle, Mégan; Leclerc, Jean-Philippe; Villemure, Elisia; Gorelsky, Serge; Fagnou, Keith (2008-03-01)."C2, C5, and C4 Azole N -Oxide Direct Arylation Including Room-Temperature Reactions".Journal of the American Chemical Society.130(11): 3276–3277.doi:10.1021/ja7107068.ISSN0002-7863.PMID18302383.
- ^Alajarín, M.; Cabrera, J.; Pastor, A.; Sánchez-Andrada, P.; Bautista, D. (2006). "On the [2+2] Cycloaddition of 2-Aminothiazoles and Dimethyl Acetylenedicarboxylate. Experimental and Computational Evidence of a Thermal Disrotatory Ring Opening of Fused Cyclobutenes".J. Org. Chem.71(14): 5328–5339.doi:10.1021/jo060664c.PMID16808523.
- ^Arduengo, A. J.; Goerlich, J. R.; Marshall, W. J. (1997). "A Stable Thiazol-2-ylidene and Its Dimer".Liebigs Annalen.1997(2): 365–374.doi:10.1002/jlac.199719970213.