Thiourea
| |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Thiourea[1] | |
| Other names
Thiocarbamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| 605327 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.494 |
| 1604 | |
| KEGG | |
PubChemCID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2811 |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| SC(NH2)2 | |
| Molar mass | 76.12g·mol−1 |
| Appearance | white solid |
| Density | 1.405 g/mL |
| Melting point | 182 °C (360 °F; 455 K) |
| 142 g/L (25 °C) | |
| −4.24×10−5cm3/mol | |
| Hazards | |
| GHSlabelling: | |
  
| |
| Warning | |
| H302,H351,H361,H411 | |
| P201,P202,P264,P270,P273,P281,P301+P312,P308+P313,P330,P391,P405,P501 | |
| NFPA 704(fire diamond) | |
| Related compounds | |
Related compounds
|
Urea Selenourea |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
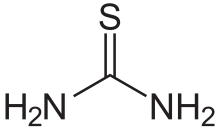
Thiourea(/ˌθaɪ.oʊjʊəˈriː.ə,-ˈjʊəri-/)[2][3][4]is anorganosulfur compoundwith theformulaSC(NH2)2and thestructureH2N−C(=S)−NH2.It is structurally similar tourea(H2N−C(=O)−NH2), except that theoxygenatom is replaced by asulfuratom (as implied by thethio-prefix); however, the properties of urea and thiourea differ significantly. Thiourea is areagentinorganic synthesis.Thioureasare a broad class of compounds with the general structureR2N−C(=S)−NR2.
Structure and bonding
[edit]Thiourea is a planar molecule. The C=S bond distance is 1.71 Å. The C-N distances average 1.33 Å.[5]The weakening of the C-S bond by C-N pi-bonding is indicated by the short C=S bond inthiobenzophenone,which is 1.63 Å.
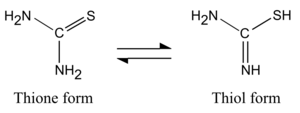
Thiourea occurs in twotautomericforms, of which thethioneform predominates in aqueous solutions. Theequilibrium constanthas been calculated asKeqis1.04×10−3.[6]Thethiolform, which is also known as an isothiourea, can be encountered in substituted compounds such asisothiouroniumsalts.
Production
[edit]The global annual production of thiourea is around 10,000 tonnes. About 40% is produced in Germany, another 40% in China, and 20% in Japan. Thiourea can be produced fromammonium thiocyanate,but more commonly it is manufactured by the reaction ofhydrogen sulfidewithcalcium cyanamidein the presence ofcarbon dioxide.[7]
- CaCN2+ 3 H2S → Ca(SH)2+ (NH2)2CS
- 2 CaCN2+ Ca(SH)2+ 6 H2O → 2 (NH2)2CS + 3 Ca(OH)2
- Ca(OH)2+ CO2→ CaCO3+ H2O
Applications
[edit]Thiox precursor
[edit]Thioureaper sehas few applications. It is mainly consumed as a precursor tothiourea dioxide,which is a common reducing agent in textile processing.[7]
Fertilizers
[edit]Recently thiourea has been investigated for its multiple desirable properties as afertilizerespecially under conditions of environmental stress.[8]It may be applied in various capacities, such as a seed pretreatment (for priming), foliar spray or medium supplementation.
Other uses
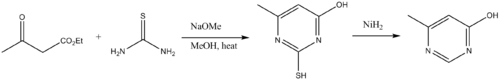
[edit]Other industrial uses of thiourea include production of flame retardant resins, andvulcanizationaccelerators. Thiourea is building blocks topyrimidinederivatives. Thus, thioureas condense with β-dicarbonyl compounds.[9]The amino group on the thiourea initially condenses with a carbonyl, followed by cyclization and tautomerization. Desulfurization delivers the pyrimidine. The pharmaceuticalsthiobarbituric acidandsulfathiazoleare prepared using thiourea.[7]4-Amino-3-hydrazino-5-mercapto-1,2,4-triazoleis prepared by the reaction of thiourea andhydrazine.
Thiourea is used as an auxiliary agent in diazo paper, light-sensitive photocopy paper and almost all other types of copy paper.
It is also used to tone silver-gelatin photographic prints (seeSepia Toning).
Thiourea is used in the Clifton-Phillips and Beaver bright and semi-bright electroplating processes.[10]It is also used in a solution with tin(II) chloride as an electroless tin plating solution for copperprinted circuit boards.
Thioureas are used (usually ashydrogen-bond donor catalysts) in a research theme calledthiourea organocatalysis.[11]Thioureas are often found to be stronger hydrogen-bond donors (i.e.,more acidic) thanureas.[12][13]
Reactions
[edit]The material has the unusual property of changing toammonium thiocyanateupon heating above130°C.Upon cooling, the ammonium salt converts back to thiourea.[citation needed]
Reductant
[edit]Thiourea reduces peroxides to the correspondingdiols.[14]The intermediate of the reaction is an unstableendoperoxide.

Thiourea is also used in the reductive workup ofozonolysisto givecarbonylcompounds.[15]Dimethyl sulfideis also an effective reagent for this reaction, but it is highly volatile (boiling point37 °C) and has an obnoxious odor whereas thiourea is odorless and conveniently non-volatile (reflecting its polarity).

Source of sulfide
[edit]Thiourea is employed as a source of sulfide, such as for convertingalkyl halidesto thiols. The reaction capitalizes on the highnucleophilicityof the sulfur center and easy hydrolysis of the intermediateisothiouronium salt:
- CS(NH2)2+ RX → RSC(NH2)+2X−
- RSC(NH2)+2X−+ 2 NaOH → RSNa + OC(NH2)2+ NaX + H2O
- RSNa + HCl → RSH + NaCl
In this example,ethane-1,2-dithiolis prepared from1,2-dibromoethane:[16]
- C2H4Br2+ 2 SC(NH2)2→ [C2H4(SC(NH2)2)2]Br2
- [C2H4(SC(NH2)2)2]Br2+ 2 KOH → C2H4(SH)2+ 2 OC(NH2)2+ 2 KBr
Like otherthioamides,thiourea can serve as a source of sulfide upon reaction with metal ions. For example,mercury sulfideforms when mercuric salts in aqueous solution are treated with thiourea:
- Hg2++ SC(NH2)2+ H2O → HgS + OC(NH2)2+ 2 H+
These sulfiding reactions, which have been applied to the synthesis of many metal sulfides, require water and typically some heating.[17][18]
Precursor to heterocycles
[edit]Thioureas are building blocks topyrimidinederivatives. Thus thioureas condense with β-dicarbonyl compounds.[19]The amino group on the thiourea initially condenses with a carbonyl, followed by cyclization and tautomerization. Desulfurization delivers the pyrimidine.
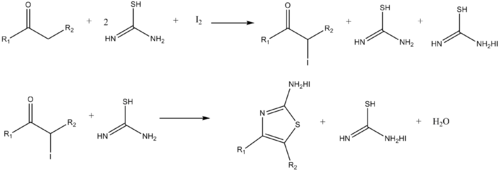
Similarly, aminothiazoles can be synthesized by the reaction of α-haloketonesand thiourea.[20]
The pharmaceuticalsthiobarbituric acidandsulfathiazoleare prepared using thiourea.[7]4-Amino-3-hydrazino-5-mercapto-1,2,4-triazoleis prepared by the reaction of thiourea andhydrazine.
Silver polishing
[edit]According to the label on consumer productsTarnX[21]andSilver Dip,[22]the liquid silver cleaning products contain thiourea along with a warning that thiourea is a chemical onCalifornia's list of carcinogens.[23]Alixiviantfor gold and silver leaching can be created by selectively oxidizing thiourea, bypassing the steps of cyanide use and smelting.[24]
Kurnakov reaction
[edit]Thiourea is an essential reagent in theKurnakov testused to differentiatecis- and trans-isomers of certain square planarplatinumcomplexes. The reaction was discovered in 1893 by Russian chemistNikolai Kurnakovand is still performed as an assay for compounds of this type.[25]
Safety
[edit]TheLD50for thiourea is125 mg/kgfor rats (oral).[26]
Agoitrogeniceffect (enlargement of the thyroid gland) has been reported for chronic exposure, reflecting the ability of thiourea to interfere with iodide uptake.[7]
A cyclic derivative of thiourea calledThiamazoleis used to treat overactive thyroid
See also
[edit]References
[edit]- ^Favre, Henri A.; Powell, Warren H. (2014).Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book).Cambridge:Royal Society of Chemistry.pp. 98, 864.doi:10.1039/9781849733069.ISBN9780854041824.OCLC1077224056.
- ^"thiourea".LexicoUK English Dictionary.Oxford University Press.Archived fromthe originalon 2020-03-22.
- ^"thiourea".Merriam-Webster Dictionary.Merriam-Webster.Retrieved2016-01-21.
- ^"thiourea".Dictionary Unabridged(Online). n.d.
- ^D. Mullen; E. Hellner (1978). "A Simple Refinement of Density Distributions of Bonding Electrons. IX. Bond Electron Density Distribution in Thiourea, CS(NH2)2,at 123K ".Acta Crystallogr.B34(9): 2789–2794.Bibcode:1978AcCrB..34.2789M.doi:10.1107/S0567740878009243.
- ^Allegretti, P.E; Castro, E.A; Furlong, J.J.P (March 2000). "Tautomeric equilibrium of amides and related compounds: theoretical and spectral evidences".Journal of Molecular Structure: THEOCHEM.499(1–3): 121–126.doi:10.1016/S0166-1280(99)00294-8.
- ^abcdeMertschenk, Bernd; Beck, Ferdinand; Bauer, Wolfgang (2002). "Thiourea and Thiourea Derivatives".Ullmann's Encyclopedia of Industrial Chemistry.Wiley-VCH.doi:10.1002/14356007.a26_803.ISBN3527306730.
- ^Wahid, Abdul (2017-08-01)."Thiourea: A Molecule with Immense Biological Significance for Plants"(PDF).International Journal of Agriculture and Biology.19(4): 911–920.doi:10.17957/ijab/15.0464.ISSN1560-8530.Archived(PDF)from the original on 2020-02-15.Retrieved2020-12-09.
- ^Foster, H. M.; Snyder, H. R. (1955). "4-Methyl-6-hydroxypyrimidine".Organic Syntheses.35:80.doi:10.15227/orgsyn.035.0080.
- ^"81st Universal Metal Finishing Guidebook".Metal Finishing, Guidebook and Directory Issue.Metal Finishing Magazine:285. Fall 2013.ISSN0026-0576.Archivedfrom the original on 2017-11-17.Retrieved2016-10-11.
- ^R. Schreiner, Peter (2003). "Metal-free organocatalysis through explicit hydrogen bonding interactions".Chem. Soc. Rev.32(5): 289–296.doi:10.1039/b107298f.PMID14518182.
- ^Jakab, Gergely; Tancon, Carlo; Zhang, Zhiguo; Lippert, Katharina M.; Schreiner, Peter R. (2012). "(Thio)urea Organocatalyst Equilibrium Acidities in DMSO".Organic Letters.14(7): 1724–1727.doi:10.1021/ol300307c.PMID22435999.
- ^Nieuwland, Celine; Fonseca Guerra, Célia (2022)."How the Chalcogen Atom Size Dictates the Hydrogen-Bond Donor Capability of Carboxamides, Thioamides, and Selenoamides".Chemistry – A European Journal.28(31): e202200755.doi:10.1002/chem.202200755.PMC9324920.PMID35322485.
- ^Kaneko, C.; Sugimoro, A.; Tanaka, S. (1974)."A facile one-step synthesis ofcis-2-cyclopentene andcis-2-cyclohexene-1,4-diols from the corresponding cyclodienes ".Synthesis.1974(12): 876–877.doi:10.1055/s-1974-23462.S2CID93207044.Archivedfrom the original on 2021-06-12.Retrieved2022-06-18.
- ^Gupta, D., Soman, G., and Dev, S. (1982). "Thiourea, a convenient reagent for the reductive cleavage of olefin ozonolysis products".Tetrahedron.38(20): 3013–3018.doi:10.1016/0040-4020(82)80187-7.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Speziale, A. J. (1963)."Ethanedithiol".Organic Syntheses;Collected Volumes,vol. 4, p. 401.
- ^Liang, Y.; et, al. (2016). "An efficient precursor to synthesize various FeS2 nanostructures via a simple hydrothermal synthesis method".CrystEngComm.18(33): 6262–6271.doi:10.1039/c6ce01203e.
- ^Bao, N.; et al. (2007). "Facile Cd−Thiourea Complex Thermolysis Synthesis of Phase-Controlled CdS Nanocrystals for Photocatalytic Hydrogen Production under Visible Light".The Journal of Physical Chemistry C.111(47): 17527–17534.doi:10.1021/jp076566s.
- ^Foster, H. M., and Snyder, H. R. (1963)."4-Methyl-6-hydroxypyrimidine".Organic Syntheses
{{cite journal}}:CS1 maint: multiple names: authors list (link);Collected Volumes,vol. 4, p. 638. - ^Dodson, R. M. & King, L. C. (1945). "The reaction of ketones with halogens and thiourea".J. Am. Chem. Soc.67(12): 2242–2243.doi:10.1021/ja01228a059.PMID21005695.
- ^"Tarn-X PRO Tarnish Remover"(PDF).The Betty Mills Company, Inc.Archived(PDF)from the original on 2021-06-06.Retrieved2021-06-06.
- ^"Hagerty Silver Dip".J.L. Smith & Co.Archivedfrom the original on 2021-06-06.Retrieved2021-06-06.
- ^Expedited Cancer Potency Values and Proposed Regulatory Levels for Certain Proposition 65 Carcinogens(PDF)(Report). April 1992.Archived(PDF)from the original on 2022-01-21.Retrieved2022-06-18.
- ^Esposito, Anthony (July 13, 2007)."Peñoles, UAM unveil pilot thiourea Au-Ag leaching plant in Mexico".Business News Americas. Archived fromthe originalon 17 February 2009.
- ^Kauffman, George B. (January 1983)."Nikolaĭ Semenovich Kurnakov, the reaction (1893) and the man (1860–1941) a ninety-year retrospective view".Polyhedron.2(9): 855–863.doi:10.1016/S0277-5387(00)81400-X.ISSN0277-5387.Archivedfrom the original on 2021-03-28.Retrieved2020-12-09.
- ^"Thiourea and its properties".September 11, 1986.Archivedfrom the original on May 27, 2010.RetrievedJanuary 6,2012.
Further reading
[edit]- Patai, Saul (1977).The Chemistry of Double-Bonded Functional Groups.New York, NY: John Wiley & Sons. pp.1355–1496.ISBN9780471924937.OCLC643207498.