Gene delivery
| Part ofa serieson |
| Genetic engineering |
|---|
 |
| Genetically modified organisms |
| History and regulation |
| Process |
| Applications |
| Controversies |
Gene deliveryis the process of introducing foreign genetic material, such asDNAorRNA,into hostcells.[1]Gene delivery must reach thegenomeof the host cell to inducegene expression.[2]Successful gene delivery requires the foreign gene delivery to remain stable within the host cell and can either integrate into thegenomeor replicate independently of it.[3]This requires foreign DNA to be synthesized as part of avector,which is designed to enter the desired host cell and deliver thetransgeneto that cell's genome.[4]Vectors utilized as the method for gene delivery can be divided into two categories, recombinant viruses and synthetic vectors (viral and non-viral).[2][5]
In complex multicellulareukaryotes(more specificallyWeissmanists), if thetransgeneis incorporated into the host'sgermlinecells, the resulting host cell can pass the transgene to itsprogeny.If the transgene is incorporated intosomaticcells, the transgene will stay with the somatic cell line, and thus its host organism.[6]
Gene delivery is a necessary step ingene therapyfor the introduction or silencing of a gene to promote a therapeutic outcome in patients and also has applications in the genetic modification of crops. There are many different methods of gene delivery for various types of cells and tissues.[6]
History
[edit]Viral based vectors emerged in the 1980s as a tool for transgene expression. In 1983, Albert Siegel described the use of viral vectors in plant transgene expression although viral manipulation via cDNA cloning was not yet available.[7]The first virus to be used as a vaccine vector was thevacciniavirus in 1984 as a way to protect chimpanzees againsthepatitis B.[8]Non-viral gene delivery was first reported on in 1943 by Avery et al. who showed cellular phenotype change viaexogenous DNAexposure.[9]
Methods
[edit]
There are a variety of methods available to deliver genes to host cells. When genes are delivered to bacteria or plants the process is calledtransformationand when it is used to deliver genes to animals it is calledtransfection.This is because transformation has adifferent meaningin relation to animals, indicating progression to a cancerous state.[10]For some bacteria no external methods are need to introduce genes as they are naturally able totake up foreign DNA.[11]Most cells require some sort of intervention to make the cell membrane permeable to DNA and allow the DNA to be stably inserted into the hostsgenome.
Chemical
[edit]Chemical based methods of gene delivery can use natural or synthetic compounds to form particles that facilitate the transfer of genes into cells.[2]These synthetic vectors have the ability to electrostatically bind DNA or RNA and compact the genetic information to accommodate larger genetic transfers.[5]Chemical vectors usually enter cells byendocytosisand can protect genetic material from degradation.[6]
Heat shock
[edit]One of the simplest method involves altering the environment of the cell and then stressing it by giving it aheat shock.Typically the cells are incubated in a solution containingdivalentcations(oftencalcium chloride) under cold conditions, before being exposed to a heat pulse. Calcium chloride partially disrupts the cell membrane, which allows the recombinant DNA to enter the host cell. It is suggested that exposing the cells to divalent cations in cold condition may change or weaken the cell surface structure, making it more permeable to DNA. The heat-pulse is thought to create a thermal imbalance across the cell membrane, which forces the DNA to enter the cells through either cell pores or the damaged cell wall.
Calcium phosphate
[edit]Another simple methods involves usingcalcium phosphateto bind the DNA and then exposing it to cultured cells. The solution, along with the DNA, isencapsulatedby the cells and a small amount of DNA can be integrated into the genome.[12]
Liposomes and polymers
[edit]Liposomesandpolymerscan be used as vectors to deliver DNA into cells. Positively charged liposomes bind with the negatively charged DNA, while polymers can be designed that interact with DNA.[2]They form lipoplexes and polyplexes respectively, which are then up-taken by the cells.[13]The two systems can also be combined.[6]Polymer-based non-viral vectors uses polymers to interact with DNA and form polyplexes.[6]
Nanoparticles
[edit]The use of engineered inorganic and organicnanoparticlesis another non-viral approach for gene delivery.[14][15]
Physical
[edit]Artificial gene delivery can be mediated by physical methods which uses force to introduce genetic material through the cell membrane.[2]
Electroporation
[edit]
Electroporationis a method of promotingcompetence.Cells are briefly shocked with anelectric fieldof 10-20kV/cm, which is thought to create holes in the cell membrane through which the plasmid DNA may enter. After the electric shock, the holes are rapidly closed by the cell's membrane-repair mechanisms.
Biolistics
[edit]
Another method used to transform plant cells isbiolistics,where particles of gold or tungsten are coated with DNA and then shot into young plant cells or plant embryos.[16]Some genetic material enters the cells and transforms them. This method can be used on plants that are not susceptible toAgrobacteriuminfection and also allows transformation of plantplastids.Plants cells can also be transformed using electroporation, which uses an electric shock to make the cell membrane permeable to plasmid DNA. Due to the damage caused to the cells and DNA the transformation efficiency of biolistics and electroporation is lower than agrobacterial transformation.[17]
Microinjection
[edit]Microinjectionis where DNA is injected through the cell'snuclear envelopedirectly into thenucleus.[11]
Sonoporation
[edit]Sonoporationis the transient permeation ofcell membranesassisted byultrasound,typically in the presence of gasmicrobubbles.[18]Sonoporation allows for the entry of genetic material into cells.[19][20]
Photoporation
[edit]Photoporationis when laser pulses are used to create pores in a cell membrane to allow entry of genetic material.
Magnetofection
[edit]Magnetofectionuses magnetic particles complexed with DNA and an external magnetic field concentrate nucleic acid particles into target cells.
Hydroporation
[edit]A hydrodynamic capillary effect can be used to manipulate cell permeability.
Agrobacterium
[edit]
In plants the DNA is often inserted usingAgrobacterium-mediated recombination,[21]taking advantage of theAgrobacteriumsT-DNAsequence that allows natural insertion of genetic material into plant cells.[22]Plant tissue are cut into small pieces and soaked in a fluid containing suspendedAgrobacterium.The bacteria will attach to many of the plant cells exposed by the cuts. The bacteria usesconjugationto transfer a DNA segment calledT-DNAfrom its plasmid into the plant. The transferred DNA is piloted to the plant cell nucleus and integrated into the host plants genomic DNA.The plasmid T-DNA is integrated semi-randomly into thegenomeof the host cell.[23]
By modifying the plasmid to express the gene of interest, researchers can insert their chosen gene stably into the plants genome. The only essential parts of the T-DNA are its two small (25 base pair) border repeats, at least one of which is needed for plant transformation.[24][25]The genes to be introduced into the plant are cloned into aplant transformation vectorthat contains the T-DNA region of theplasmid.An alternative method isagroinfiltration.[26][27]
Viral delivery
[edit]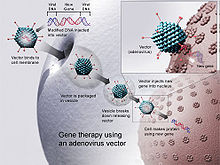
Virusmediated gene delivery utilizes the ability of a virus to inject its DNA inside a host cell and takes advantage of the virus' own ability to replicate and implement their own genetic material. Viral methods of gene delivery are more likely to induce an immune response, but they have high efficiency.[6]Transductionis the process that describes virus-mediated insertion of DNA into the host cell. Viruses are a particularly effective form of gene delivery because the structure of the virus prevents degradation vialysosomesof the DNA it is delivering to the nucleus of the host cell.[28]In gene therapy a gene that is intended for delivery is packaged into a replication-deficient viral particle to form aviral vector.[29]Viruses used for gene therapy to date include retrovirus, adenovirus, adeno-associated virus and herpes simplex virus. However, there are drawbacks to using viruses to deliver genes into cells. Viruses can only deliver very small pieces of DNA into the cells, it is labor-intensive and there are risks of random insertion sites,cytopathic effectsand mutagenesis.[30]
Viral vector based gene delivery uses aviral vectorto deliver genetic material to the host cell. This is done by using a virus that contains the desired gene and removing the part of the viruses genome that is infectious.[2]Viruses are efficient at delivering genetic material to the host cell's nucleus, which is vital for replication.[2]
RNA-based viral vectors
[edit]RNA-based viruses were developed because of the ability to transcribe directly from infectious RNA transcripts. RNA vectors are quickly expressed and expressed in the targeted form since no processing is required [source needed]. Retroviral vectors include oncoretroviral,lentiviralandhuman foamy virusare RNA-based viral vectors that reverse transcript and integrated into the host genome, permits long-term transgene expression.[2]
DNA-based viral vectors
[edit]DNA-based viral vectors includeAdenoviridae,adeno-associated virusandherpes simplex virus.[2]
Applications
[edit]Gene therapy
[edit]Several of the methods used to facilitate gene delivery have applications for therapeutic purposes.Gene therapyutilizes gene delivery to deliver genetic material with the goal of treating a disease or condition in the cell. Gene delivery in therapeutic settings utilizes non-immunogenicvectors capable of cell specificity that can deliver an adequate amount of transgene expression to cause the desired effect.[3]
Advances ingenomicshave enabled a variety of new methods and gene targets to be identified for possible applications.DNA microarraysused in a variety ofnext-gen sequencingcan identify thousands of genes simultaneously, with analytical software looking at gene expression patterns, andorthologousgenes inmodel speciesto identify function.[31]This has allowed a variety of possible vectors to be identified for use in gene therapy. As a method for creating a new class of vaccine, gene delivery has been utilized to generate ahybridbiosynthetic vector to deliver a possible vaccine. This vector overcomes traditional barriers to gene delivery by combiningE. coliwith asynthetic polymerto create a vector that maintains plasmid DNA while having an increased ability to avoid degradation by target cell lysosomes.[32]
See also
[edit]References
[edit]- ^Jones CH, Chen CK, Ravikrishnan A, Rane S, Pfeifer BA (November 2013)."Overcoming nonviral gene delivery barriers: perspective and future".Molecular Pharmaceutics.10(11): 4082–98.doi:10.1021/mp400467x.PMC5232591.PMID24093932.
- ^abcdefghiKamimura K, Suda T, Zhang G, Liu D (October 2011)."Advances in Gene Delivery Systems".Pharmaceutical Medicine.25(5): 293–306.doi:10.1007/bf03256872.PMC3245684.PMID22200988.
- ^abMali S (January 2013)."Delivery systems for gene therapy".Indian Journal of Human Genetics.19(1): 3–8.doi:10.4103/0971-6866.112870.PMC3722627.PMID23901186.
- ^Gibson G, Muse SV (2009).A Primer of Genome Science(Third ed.). 23 Plumtree Rd, Sunderland, MA 01375: Sinauer Associates. pp. 304–305.ISBN978-0-87893-236-8.
{{cite book}}:CS1 maint: location (link) - ^abPack DW, Hoffman AS, Pun S, Stayton PS (July 2005). "Design and development of polymers for gene delivery".Nature Reviews. Drug Discovery.4(7): 581–93.doi:10.1038/nrd1775.PMID16052241.S2CID20972049.
- ^abcdefNayerossadat N, Maedeh T, Ali PA (6 July 2012)."Viral and nonviral delivery systems for gene delivery".Advanced Biomedical Research.1:27.doi:10.4103/2277-9175.98152.PMC3507026.PMID23210086.
- ^Yusibov V, Shivprasad S, Turpen TH, Dawson W, Koprowski H (1999). "Plant Viral Vectors Based on Tobamoviruses".Plant Biotechnology.Current Topics in Microbiology and Immunology. Vol. 240. pp. 81–94.doi:10.1007/978-3-642-60234-4_4.ISBN978-3-540-66265-5.PMID10394716.
- ^Moss B, Smith GL, Gerin JL, Purcell RH (September 1984)."Live recombinant vaccinia virus protects chimpanzees against hepatitis B".Nature.311(5981): 67–9.Bibcode:1984Natur.311...67M.doi:10.1038/311067a0.PMID6472464.S2CID4358204.
- ^Avery OT, MacLeod CM, McCarty M (2017).Die Entdeckung der Doppelhelix.Klassische Texte der Wissenschaft (in German). Springer Spektrum, Berlin, Heidelberg. pp. 97–120.doi:10.1007/978-3-662-47150-0_2.ISBN9783662471494.S2CID52805314.
- ^Alberts B,Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002).Molecular Biology of the Cell.New York: Garland Science. p. G:35.ISBN978-0-8153-4072-0.
- ^abChen I, Dubnau D (March 2004). "DNA uptake during bacterial transformation".Nature Reviews. Microbiology.2(3): 241–9.doi:10.1038/nrmicro844.PMID15083159.S2CID205499369.
- ^"Lecture 8 genetic engineering of animal cells".slideshare.net.2012-01-25.Retrieved2018-07-18.
- ^Biocyclopedia."Gene transfer (transfection) methods in animals | Genetic Engineering and Biotechnology Gene Transfer Methods and Transgenic Organisms | Genetics, Biotechnology, Molecular Biology, Botany | Biocyclopedia".biocyclopedia.Retrieved2018-07-18.
- ^Yin, Feng; Gu, Bobo; Lin, Yining; Panwar, Nishtha; Tjin, Swee Chuan; Qu, Junle; Lau, Shu Ping; Yong, Ken-Tye (15 September 2017). "Functionalized 2D nanomaterials for gene delivery applications".Coordination Chemistry Reviews.347:77.doi:10.1016/j.ccr.2017.06.024.hdl:10397/95016.
- ^Singh BN, Prateeksha, Gupta VK, Chen J, Atanasov AG.Organic Nanoparticle-Based Combinatory Approaches for Gene Therapy.Trends Biotechnol. 2017 Dec;35(12):1121–1124.doi: 10.1016/j.tibtech.2017.07.010.
- ^Head G, Hull RH, Tzotzos GT (2009).Genetically Modified Plants: Assessing Safety and Managing Risk.London: Academic Pr. p. 244.ISBN978-0-12-374106-6.
- ^Hwang, HH; Yu, M; Lai, EM (2017)."Agrobacterium-mediated plant transformation: biology and applications".The Arabidopsis Book.15:e0186.doi:10.1199/tab.0186.PMC6501860.PMID31068763.
- ^Postema M, Kotopoulis S, Delalande A, Gilja OH (2012)."Sonoporation: why microbubbles create pores".Ultraschall in der Medizin.33(1): 97–98.doi:10.1055/s-0031-1274749.S2CID260344222.
- ^Delalande A, Postema M, Mignet N, Midoux P, Pichon C (2012)."Ultrasound and microbubble-assisted gene delivery: recent advances and ongoing challenges".Therapeutic Delivery.3(10): 1199–1215.doi:10.4155/TDE.12.100.PMID23116012.S2CID20924113.
- ^Delalande A, Kotopoulis S, Postema M, Midoux P, Pichon C (2013)."Sonoporation: mechanistic insights and ongoing challenges for gene transfer".Gene.525(2): 191–199.doi:10.1016/j.gene.2013.03.095.PMID23566843.
- ^National Research Council (US) Committee on Identifying and Assessing Unintended Effects of Genetically Engineered Foods on Human Health (2004-01-01).Methods and Mechanisms for Genetic Manipulation of Plants, Animals, and Microorganisms.National Academies Press (US).
- ^Gelvin SB (March 2003)."Agrobacterium-mediated plant transformation: the biology behind the" gene-jockeying "tool".Microbiology and Molecular Biology Reviews.67(1): 16–37, table of contents.doi:10.1128/MMBR.67.1.16-37.2003.PMC150518.PMID12626681.
- ^Francis KE, Spiker S (February 2005)."Identification of Arabidopsis thaliana transformants without selection reveals a high occurrence of silenced T-DNA integrations".The Plant Journal.41(3): 464–77.doi:10.1111/j.1365-313x.2004.02312.x.PMID15659104.
- ^Schell J, Van Montagu M (1977). "The Ti-Plasmid of Agrobacterium Tumefaciens, A Natural Vector for the Introduction of NIF Genes in Plants?". In Hollaender A, Burris RH, Day PR, Hardy RW, Helinski DR, Lamborg MR, Owens L, Valentine RC (eds.).Genetic Engineering for Nitrogen Fixation.Basic Life Sciences. Vol. 9. pp. 159–79.doi:10.1007/978-1-4684-0880-5_12.ISBN978-1-4684-0882-9.PMID336023.
- ^Joos H, Timmerman B, Montagu MV, Schell J (1983)."Genetic analysis of transfer and stabilization of Agrobacterium DNA in plant cells".The EMBO Journal.2(12): 2151–60.doi:10.1002/j.1460-2075.1983.tb01716.x.PMC555427.PMID16453483.
- ^Thomson JA."Genetic Engineering of Plants"(PDF).Biotechnology.3.Retrieved17 July2016.
- ^Leuzinger K, Dent M, Hurtado J, Stahnke J, Lai H, Zhou X, Chen Q (July 2013)."Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins".Journal of Visualized Experiments.77(77).doi:10.3791/50521.PMC3846102.PMID23913006.
- ^Wivel NA, Wilson JM (June 1998)."Methods of gene delivery".Hematology/Oncology Clinics of North America.12(3): 483–501.doi:10.1016/s0889-8588(05)70004-6.PMID9684094.
- ^Lodish H, Berk A, Zipursky SL, et al. (2000).Molecular Cell Biology(Fourth ed.). New York: W. H. Freeman and Company. pp. Section 6.3, Viruses: Structure, Function, and Uses.ISBN9780716737063.
- ^Keles E, Song Y, Du D, Dong WJ, Lin Y (August 2016). "Recent progress in nanomaterials for gene delivery applications".Biomaterials Science.4(9): 1291–309.doi:10.1039/C6BM00441E.PMID27480033.
- ^Guyon I, Weston J, Barnhill S, Vapnik V (2002)."Gene Selection for Cancer Classification using Support Vector Machines".Machine Learning.46:389–422.doi:10.1023/A:1012487302797.
- ^Jones CH, Ravikrishnan A, Chen M, Reddinger R, Kamal Ahmadi M, Rane S, Hakansson AP, Pfeifer BA (August 2014)."Hybrid biosynthetic gene therapy vector development and dual engineering capacity".Proceedings of the National Academy of Sciences of the United States of America.111(34): 12360–5.Bibcode:2014PNAS..11112360J.doi:10.1073/pnas.1411355111.PMC4151754.PMID25114239.
Further reading
[edit]- Segura T, Shea LD (2001). "Materials for non-viral gene delivery".Annual Review of Materials Research.31:25–46.Bibcode:2001AnRMS..31...25S.doi:10.1146/annurev.matsci.31.1.25.
- Luo D, Saltzman WM (January 2000). "Synthetic DNA delivery systems".Nature Biotechnology.18(1): 33–7.doi:10.1038/71889.PMID10625387.S2CID7068508.
