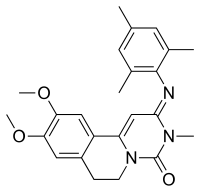
Trequinsin
Appearance
| |
| Names | |
|---|---|
| Preferred IUPAC name
9,10-Dimethoxy-3-methyl-2-[(2,4,6-trimethylphenyl)imino]-2,3,6,7-tetrahydro-4H-pyrimido[6,1-a]isoquinolin-4-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C24H27N3O3 | |
| Molar mass | 405.498g·mol−1 |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Trequinsinis aphosphodiesterase inhibitor.[1]It has been shown to improvespermmotilityin vitro.[2]
References[edit]
- ^Whitaker, RM; Wills, LP; Stallons, LJ; Schnellmann, RG (2013)."CGMP-Selective Phosphodiesterase Inhibitors Stimulate Mitochondrial Biogenesis and Promote Recovery from Acute Kidney Injury".The Journal of Pharmacology and Experimental Therapeutics.347(3): 626–34.doi:10.1124/jpet.113.208017.PMC3836317.PMID24042162.
- ^McBrinn RC, Fraser J, Hope AG, Gray DW, Barratt CLR, Martins da Silva SJ, Brown SG. Novel pharmacological actions of trequinsin hydrochloride improve human sperm cell motility and function.Br J Pharmacol.2019 Dec;176(23):4521-4536.doi:10.1111/bph.14814PMID31368510
