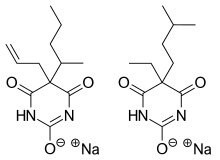
Tuinal
 structure | |
| Combination of | |
|---|---|
| secobarbital | short-acting barbiturate |
| amobarbital | intermediate-acting barbiturate |
| Clinical data | |
| Routes of administration | oral |
Tuinalwas the brand name of a discontinued combinationdrugcomposed of twobarbituratesalts (secobarbitalsodium andamobarbitalsodium) in equal proportions.
Tuinal was introduced as asedative-hypnotic(sleeping pill) medication in the late 1940s byEli Lilly.It was also used in obstetrics for childbirth.[1][2]It was produced in brightly colored half-reddish orange and half-turquoise bluegelatin capsuleform (bullet-shaped Pulvules) for oral administration. Individual capsules contained 50 mg, 100 mg, or 200 mg of barbiturate salts. The combination of a short-acting barbiturate, Secobarbital, with an intermediate-acting barbiturate, Amobarbital, aimed to provide "a rapid yet prolonged hypnotic action".[3]
Eli Lilly has discontinued the manufacture of Tuinal in the United States due to the diminishing use of barbiturates (replaced by thebenzodiazepinefamily of drugs) in outpatient treatment, and its widespread abuse.[4]Currently, Valleant Labs markets Secobarbital capsules only. Flynn Pharma of Ireland no longer manufactures Tuinal,Seconalor Amytal (amobarbital). Amytal has been discontinued, though sodium amytal injection form remains.
Abuse
[edit]
Tuinal saw widespread abuse as arecreational drugfrom the 1960s through the 1980s. The pill was known colloquially under the street names "tuies", "tumies", "double trouble", "blue tips", "F-66's" (which were the markings on Lilly's capsule), "rainbows", "beans", "nawls" and "jeebs".[5]It came in the form of bullet-shaped capsules, half-reddish orange and half-turquoise blue. Like other barbituratedepressants,Tuinal promotes physical and psychologicaldependencybeginning after one week of regular use and carries a high risk ofoverdose.[6]It was reported in the 1980s as one of the most common ways ofself-poisoning.[7]Abuse of this particular drug tapered off after it was discontinued by manufacturers in the late 1990s.
Tuinal is classified as a Schedule II drug under theControlled Substances Actin the United States, meaning it requires a prescription from a licensed practitioner.
References
[edit]- ^Waters AB (1947)."Pethidine In Labour".The British Medical Journal.2(4514): 71–72.doi:10.1136/bmj.2.4514.71-b.ISSN0007-1447.JSTOR20370143.PMC2055200.PMID20344014.
- ^"Front Matter".The British Medical Journal.1(4539). 1948.ISSN0007-1447.JSTOR25361874.
- ^"Front Matter".The American Journal of Nursing.47(5): 1–24. 1947.ISSN0002-936X.JSTOR3457169.
- ^Mitchell M, Willingham EJ, Atkins WA (2012). Key K (ed.).The Gale Encyclopedia of Mental Health.Vol. 1 (3rd ed.). Detroit, MI: Gale eBooks. p. 171.ISBN9781414490144.RetrievedNovember 4,2022.
- ^Bigelow BC, ed. (2006).UXL Encyclopedia of Drugs and Addictive Substances.Detroit, MI: Gale eBooks. p. 99.ISBN9781414404448.RetrievedNovember 4,2022.
- ^Evans JI, Lewis SA, Gibb IA, Cheetham M (November 1968)."Sleep and birbiturates: some experiments and observations".British Medical Journal.4(5626): 291–293.doi:10.1136/bmj.4.5626.291.PMC1912258.PMID4301261.
- ^Ray JE, Reilly DK, Day RO (April 1986)."Drugs involved in self-poisoning: verification by toxicological analysis".The Medical Journal of Australia.144(9): 455–457.doi:10.5694/j.1326-5377.1986.tb101047.x.PMID2871482.S2CID24568454.
