Allotropes of phosphorus


Elementalphosphoruscan exist in severalallotropes,the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists asdiphosphorusand atomic phosphorus.
White phosphorus[edit]
 White phosphorus sample with a chunk removed from the corner to expose un-oxidized material
| |
 Tetraphosphorus molecule
| |
| Names | |
|---|---|
| IUPAC names
White phosphorus
Tetraphosphorus | |
| Systematic IUPAC name
1,2,3,4-Tetraphosphatricyclo[1.1.0.02,4]butane | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChemCID
|
|
| UN number | 1381 |
| |
| |
| Properties | |
| P4 | |
| Molar mass | 123.895g·mol−1 |
| Density | 1.82 g/cm3 |
| Melting point | 44.1 °C; 111.4 °F; 317.3 K |
| Boiling point | 280 °C; 536 °F; 553 K |
| Hazards | |
| NFPA 704(fire diamond) | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
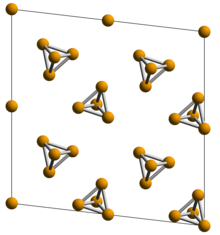
White phosphorus,yellow phosphorusor simplytetraphosphorus(P4) exists asmoleculesof four phosphorusatomsin a tetrahedral structure, joined by six phosphorus—phosphorussingle bonds.Thetetrahedralarrangement results inring strainand instability.[1]Although both are called "white phosphorus", in fact two different crystal allotropes are known, interchanging reversibly at 195.2 K.[2]The element'sstandard stateis thebody-centered cubicα form, which is actuallymetastableunderstandard conditions.[1]The β form is believed to have ahexagonalcrystal structure.[2]
Molten and gaseous white phosphorus also retains the tetrahedral molecules, until 800 °C (1,500 °F; 1,100 K) when it starts decomposing toP
2molecules.[3]TheP
4molecule in the gas phase has a P-P bond length ofrg= 2.1994(3) Å as was determined bygas electron diffraction.[4]The β form of white phosphorus contains three slightly differentP
4molecules, i.e. 18 different P-P bond lengths — between 2.1768(5) and 2.1920(5) Å. The average P-P bond length is 2.183(5) Å.[3]
White phosphorus is a translucentwaxysolid that quickly yellows in light, and impure white phosphorus is for this reason called yellow phosphorus. It glows greenish in the dark (when exposed to oxygen) and is highlyflammableandpyrophoric(self-igniting) upon contact with air. It istoxic,causing severeliver damageon ingestion andphossy jawfrom chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic smell, and samples are commonly coated with white "diphosphorus pentoxide",which consists ofP4O10tetrahedral with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can be stored under water. Indeed, white phosphorus is safe from self-igniting when it is submerged in water; due to this, unreacted white phosphorus can prove hazardous tobeachcomberswho may collect washed-up samples while unaware of their true nature.[5][6]P4is soluble inbenzene,oils,carbon disulfide,anddisulfur dichloride.
Production and applications[edit]
The white allotrope can be produced using several methods. In the industrial process,phosphate rockis heated in an electric or fuel-firedfurnacein the presence ofcarbonandsilica.[7]Elemental phosphorus is then liberated as a vapour and can be collected underphosphoric acid.An idealized equation for thiscarbothermal reactionis shown forcalcium phosphate(although phosphate rock contains substantial amounts offluoroapatite):
- 2 Ca3(PO4)2+ 6 SiO2+ 10 C → 6 CaSiO3+ 10 CO + P4
White phosphorus has an appreciablevapour pressureat ordinary temperatures. Thevapour densityindicates that the vapour is composed ofP4molecules up to about 800 °C. Above that temperature, dissociation intoP2molecules occurs.
Inbase,white phosphorus spontaneouslydisproportionatestophosphineand various phosphorusoxyacids.[8]
It ignites spontaneously in air at about 50 °C (122 °F), and at much lower temperatures if finely divided (due tomelting-point depression). Phosphorus reacts with oxygen, usually formingtwooxides depending on the amount of available oxygen:P4O6(phosphorus trioxide) when reacted with a limited supply of oxygen, andP4O10when reacted with excess oxygen. On rare occasions,P4O7,P4O8,andP4O9are also formed, but in small amounts. This combustion gives phosphorus(V) oxide:
- P4+ 5 O2→ P4O10
Because of this property,white phosphorus is used as a weapon.
Phosphorus pentachlorideis prepared by the reaction of white phosphorus with excess of drychlorine.[9]
- P4+ 10Cl2→ 4PCl5
It can also be prepared by the action ofsulfuryl chlorideon white phosphorus.[9]
- P4+ 10SO2Cl2→ 4PCl5+ 10SO2
Other polyhedrane analogues[edit]
Although white phosphorus forms thetetrahedron,the simplest possiblePlatonic hydrocarbon,no other polyhedral phosphorus clusters are known.[10]White phosphorus converts to the thermodynamically-stabler red allotrope, but that allotrope is not isolated polyhedra.
Cubane,in particular, is unlikely to form,[10]and the closest approach is the half-phosphorus compoundP4(CH)4,produced fromphosphaalkynes.[11]Other clusters are more thermodynamically favorable, and some have been partially formed as components of larger polyelemental compounds.[10]
Red phosphorus[edit]


Red phosphorusmay be formed by heatingwhite phosphorusto 300 °C (570 °F) in the absence of air or by exposing white phosphorus tosunlight.Red phosphorus exists as anamorphousnetwork. Upon further heating, the amorphous red phosphorus crystallizes. Bulk red phosphorus does not ignite in air at temperatures below 240 °C (460 °F), whereas pieces of white phosphorus ignite at about 30 °C (86 °F).
Under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically stable black phosphorus. Thestandard enthalpy of formationof red phosphorus is −17.6 kJ/mol.[1]Red phosphorus is kinetically most stable.
It was first presented by Anton von Schrötter before the Vienna Academy of Sciences on December 9, 1847, although others had doubtlessly had this substance in their hands before, such as Berzelius.[12]
Applications[edit]
Red phosphorus can be used as a very effectiveflame retardant,especially inthermoplastics(e.g.polyamide) andthermosets(e.g.epoxy resinsorpolyurethanes). The flame retarding effect is based on the formation ofpolyphosphoric acid.Together with the organic polymer material, these acids create a char that prevents the propagation of the flames. The safety risks associated withphosphinegeneration andfriction sensitivityof red phosphorus can be effectively minimized by stabilization andmicro-encapsulation.For easier handling, red phosphorus is often used in form of dispersions or masterbatches in various carrier systems. However, for electronic/electrical systems, red phosphorus flame retardant has been effectively banned by major OEMs due to its tendency to induce premature failures.[13]One persistent problem is that red phosphorus in epoxy molding compounds induces elevated leakage current in semiconductor devices.[14]Another problem was acceleration ofhydrolysisreactions inPBTinsulating material.[15]
Red phosphorus can also be used in the illicit production ofmethamphetamineandKrokodil.
Red phosphorus can be used as an elementalphotocatalystfor hydrogen formation from the water.[16]They display a steady hydrogen evolution rates of 633 μmol/(h⋅g) by the formation of small-sized fibrous phosphorus.[17]
Violet or Hittorf's phosphorus[edit]

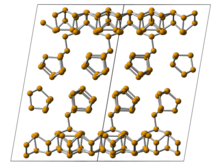
Monoclinic phosphorus,orviolet phosphorus,is also known asHittorf's metallic phosphorus.[18][19]In 1865,Johann Wilhelm Hittorfheated red phosphorus in a sealed tube at 530 °C. The upper part of the tube was kept at 444 °C. Brilliant opaquemonoclinic,orrhombohedral,crystals sublimed as a result. Violet phosphorus can also be prepared by dissolving white phosphorus in moltenleadin a sealed tube at 500 °C for 18 hours. Upon slow cooling, Hittorf's allotropecrystallisesout. The crystals can be revealed by dissolving the lead in dilutenitric acidfollowed by boiling in concentratedhydrochloric acid.[20]In addition, afibrousform exists with similar phosphorus cages. The lattice structure of violet phosphorus was presented by Thurn and Krebs in 1969.[21]Imaginary frequencies, indicating the irrationalities or instabilities of the structure, were obtained for the reported violet structure from 1969.[22]The single crystal of violet phosphorus was also produced. The lattice structure of violet phosphorus has been obtained by single-crystalx-ray diffraction to be monoclinic with space group ofP2/n(13) (a= 9.210,b= 9.128,c= 21.893 Å,β= 97.776°,CSD-1935087). The optical band gap of the violet phosphorus was measured by diffuse reflectance spectroscopy to be around 1.7 eV. The thermal decomposition temperature was 52 °C higher than its black phosphorus counterpart. The violet phosphorene was easily obtained from both mechanical and solution exfoliation.
Reactions of violet phosphorus[edit]
Violet phosphorus does not ignite in air until heated to 300 °C and is insoluble in all solvents. It is not attacked byalkaliand only slowly reacts withhalogens.It can beoxidisedbynitric acidtophosphoric acid.
If it is heated in an atmosphere of inert gas, for examplenitrogenorcarbon dioxide,itsublimesand the vapour condenses as white phosphorus. If it is heated in avacuumand the vapour condensed rapidly, violet phosphorus is obtained. It would appear that violet phosphorus is apolymerof high relative molecular mass, which on heating breaks down intoP2molecules. On cooling, these would normallydimerizeto giveP4molecules (i.e. white phosphorus) but, in avacuum,they link up again to form the polymeric violet allotrope.
Black phosphorus[edit]


Black phosphorusis the thermodynamically stable form of phosphorus atroom temperature and pressure,with aheat of formationof −39.3 kJ/mol (relative to white phosphorus which is defined as the standard state).[1]It was first synthesized by heating white phosphorus under high pressures (12,000 atmospheres) in 1914. As a 2D material, in appearance, properties, and structure, black phosphorus is very much likegraphitewith both being black and flaky, a conductor of electricity, and having puckered sheets of linked atoms.[23]
Black phosphorus has anorthorhombicpleated honeycomb structure and is the least reactive allotrope, a result of its lattice of interlinked six-membered rings where each atom is bonded to three other atoms.[24][25]In this structure, each phosphorus atom has five outer shell electrons.[26]Black and red phosphorus can also take acubiccrystal lattice structure.[27]The first high-pressure synthesis of black phosphorus crystals was made by the Nobel prize winnerPercy Williams Bridgmanin 1914.[28]Metal salts catalyze the synthesis of black phosphorus.[29]
Black phosphorus based sensors exhibit several superior qualities over traditional materials used in piezoelectric or resistive sensors. Characterized by its unique puckered honeycomb lattice structure, black phosphorus provides exceptional carrier mobility. This property ensures its high sensitivity and mechanical resilience, making it an intriguing candidate forsensor technology.[30][31]
Phosphorene[edit]
The similarities to graphite also include the possibility of scotch-tape delamination (exfoliation), resulting inphosphorene,agraphene-like 2D material with excellent charge transport properties, thermal transport properties and optical properties. Distinguishing features of scientific interest include a thickness dependent band-gap, which is not found in graphene.[32]This, combined with a high on/off ratio of ~105makes phosphorene a promising candidate for field-effect transistors (FETs).[33]The tunable bandgap also suggests promising applications in mid-infrared photodetectors and LEDs.[34][35]Exfoliated black phosphorus sublimes at 400 °C in vacuum.[36]It gradually oxidizes when exposed to water in the presence of oxygen, which is a concern when contemplating it as a material for the manufacture of transistors, for example.[37][38]Exfoliated black phosphorus is an emerging anode material in the battery community, showing high stability andlithiumstorage.[39]
Ring-shaped phosphorus[edit]
Ring-shaped phosphorus was theoretically predicted in 2007.[40]The ring-shaped phosphorus was self-assembled inside evacuated multi-walled carbon nanotubes with inner diameters of 5–8 nm using a vapor encapsulation method. A ring with a diameter of 5.30 nm, consisting of 23P8and 23P2units with a total of 230 P atoms, was observed inside a multi-walled carbon nanotube with an inner diameter of 5.90 nm in atomic scale. The distance between neighboring rings is 6.4 Å.[41]
TheP6ring shaped molecule is not stable in isolation.
Blue phosphorus[edit]
Single-layer blue phosphorus was first produced in 2016 by the method ofmolecular beam epitaxyfrom black phosphorus as precursor.[42]
Diphosphorus[edit]


Thediphosphorusallotrope (P2) can normally be obtained only under extreme conditions (for example, fromP4at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use oftransition metalcomplexes(for example,tungstenandniobium).[43]
Diphosphorus is the gaseous form ofphosphorus,and the thermodynamically stable form between 1200 °C and 2000 °C. The dissociation of tetraphosphorus (P4) begins at lower temperature: the percentage ofP2at 800 °C is ≈ 1%. At temperatures above about 2000 °C, the diphosphorus molecule begins to dissociate into atomic phosphorus.
Phosphorus nanorods[edit]
P12nanorodpolymers were isolated from CuI-P complexes using low temperature treatment.[44]
Red/brown phosphorus was shown to be stable in air for several weeks and have properties distinct from those of red phosphorus.Electron microscopyshowed that red/brown phosphorus forms long, parallel nanorods with a diameter between 3.4Åand 4.7 Å.[44]
Properties[edit]
| Form | white(α) | white(β) | violet | black |
|---|---|---|---|---|
| Symmetry | Body-centred cubic | Triclinic | Monoclinic | Orthorhombic |
| Pearson symbol | aP24 | mP84 | oS8 | |
| Space group | I43m | P1No. 2 | P2/c No. 13 | Cmca No. 64 |
| Density(g/cm3) | 1.828 | 1.88 | 2.36 | 2.69 |
| Bandgap(eV) | 2.1 | 1.5 | 0.34 | |
| Refractive index | 1.8244 | 2.6 | 2.4 |
See also[edit]
References[edit]
- ^abcdHousecroft, C. E.; Sharpe, A. G. (2004).Inorganic Chemistry(2nd ed.). Prentice Hall. p. 392.ISBN978-0-13-039913-7.
- ^abDurif, A.; Averbuch-Pouchot, M.T. (1996).Topics in phosphate chemistry.Singapore [u.a.]: World Scientific. p. 3.ISBN978-981-02-2634-3.
- ^abSimon, Arndt; Borrmann, Horst; Horakh, Jörg (1997). "On the Polymorphism of White Phosphorus".Chemische Berichte.130(9): 1235–1240.doi:10.1002/cber.19971300911.
- ^Cossairt, Brandi M.; Cummins, Christopher C.; Head, Ashley R.; Lichtenberger, Dennis L.; Berger, Raphael J. F.; Hayes, Stuart A.; Mitzel, Norbert W.; Wu, Gang (2010-06-01). "On the Molecular and Electronic Structures of AsP3 and P4".Journal of the American Chemical Society.132(24): 8459–8465.doi:10.1021/ja102580d.ISSN0002-7863.PMID20515032.
- ^"A dangerous guide to beachcombing".
- ^"Woman mistakes WWII-era munition for precious stone on German beach | DW | 05.08.2017".Deutsche Welle.
- ^Threlfall, R.E., (1951).100 years of Phosphorus Making: 1851–1951.Oldbury:Albright and WilsonLtd
- ^Engel, Robert; Cohen, JaimeLee Iolani (2004).Synthesis of Carbon-Phosphorus Bonds(2nd ed.). Boca Raton:CRC Press.§2.3.ISBN0-8493-1617-0.LCCN2003060796.
- ^abChemistry Part I Class XII(PDF)(Reprinted ed.). India: NCERT. January 2019. p. 177.ISBN81-7450-648-9.
- ^abcCorbridge, D. E. C. (1995) "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam. § 4.1.12.ISBN0-444-89307-5.
- ^Streubel, Rainer (1995). "Phosphaalkyne Cyclooligomers: From Dimers to Hexamers—First Steps on the Way to Phosphorus–Carbon Cage Compounds".Angewandte Chemie International Edition in English.34(4): 436–438.doi:10.1002/anie.199504361.
- ^Kohn, Moritz (1944-11-01)."The discovery of red phosphorus (1847) by Anton von Schrötter (1802–1875)".Journal of Chemical Education.21(11): 522.Bibcode:1944JChEd..21..522K.doi:10.1021/ed021p522.ISSN0021-9584.
- ^"Red Phosphorus Reliability Alert"(PDF).Archived fromthe original(PDF)on 2018-01-02.Retrieved2018-01-01.
- ^Craig Hillman, Red Phosphorus Induced Failures in Encapsulated Circuits,https:// dfrsolutions /hubfs/Resources/services/Red-Phosphorus-Induced-Failures-in-Encapsulated-Circuits.pdf?t=1513022462214
- ^Dock Brown, The Return of the Red Retardant, SMTAI 2015,https:// dfrsolutions /hubfs/Resources/services/The-Return-of-the-Red-Retardant.pdf?t=1513022462214
- ^Applied Catalysis B: Environmental, 2012, 111–112, 409–414.
- ^Angewandte Chemie International Edition, 2016, 55, 9580–9585.
- ^Curry, Roger (2012-07-08)."Hittorf's Metallic Phosphorus of 1865".LATERAL SCIENCE.Retrieved16 November2014.
- ^Monoclinic phosphorus formed from vapor in the presence of an alkali metalU.S. patent 4,620,968
- ^Hittorf, W. (1865)."Zur Kenntniss des Phosphors".Annalen der Physik.202(10): 193–228.Bibcode:1865AnP...202..193H.doi:10.1002/andp.18652021002.
- ^Thurn, H.; Krebs, H. (1969-01-15). "Über Struktur und Eigenschaften der Halbmetalle. XXII. Die Kristallstruktur des Hittorfschen Phosphors".Acta Crystallographica Section B(in German).25(1): 125–135.Bibcode:1969AcCrB..25..125T.doi:10.1107/S0567740869001853.ISSN0567-7408.
- ^Zhang, Lihui; Huang, Hongyang; Zhang, Bo; Gu, Mengyue; Zhao, Dan; Zhao, Xuewen; Li, Longren; Zhou, Jun; Wu, Kai; Cheng, Yonghong; Zhang, Jinying (2020). "Structure and Properties of Violet Phosphorus and Its Phosphorene Exfoliation".Angewandte Chemie.132(3): 1090–1096.Bibcode:2020AngCh.132.1090Z.doi:10.1002/ange.201912761.ISSN1521-3757.PMID31713959.S2CID241932000.
- ^Korolkov, Vladimir V.; Timokhin, Ivan G.; Haubrichs, Rolf; Smith, Emily F.; Yang, Lixu; Yang, Sihai; Champness, Neil R.; Schröder, Martin; Beton, Peter H. (2017-11-09)."Supramolecular networks stabilise and functionalise black phosphorus".Nature Communications.8(1): 1385.Bibcode:2017NatCo...8.1385K.doi:10.1038/s41467-017-01797-6.ISSN2041-1723.PMC5680224.PMID29123112.
- ^Brown, A.; Rundqvist, S. (1965). "Refinement of the crystal structure of black phosphorus".Acta Crystallographica.19(4): 684–685.Bibcode:1965AcCry..19..684B.doi:10.1107/S0365110X65004140.
- ^Cartz, L.; Srinivasa, S. R.; Riedner, R. J.; Jorgensen, J. D.; Worlton, T. G. (1979). "Effect of pressure on bonding in black phosphorus".The Journal of Chemical Physics.71(4): 1718.Bibcode:1979JChPh..71.1718C.doi:10.1063/1.438523.
- ^Ling, Xi; Wang, Han; Huang, Shengxi; Xia, Fengnian; Dresselhaus, Mildred S. (2015-03-27)."The renaissance of black phosphorus".Proceedings of the National Academy of Sciences.112(15): 4523–4530.arXiv:1503.08367.Bibcode:2015PNAS..112.4523L.doi:10.1073/pnas.1416581112.ISSN0027-8424.PMC4403146.PMID25820173.
- ^Ahuja, Rajeev (2003). "Calculated high pressure crystal structure transformations for phosphorus".Physica Status Solidi B.235(2): 282–287.Bibcode:2003PSSBR.235..282A.doi:10.1002/pssb.200301569.S2CID120578034.
- ^Bridgman, P. W. (1914-07-01)."Two New Modifications of Phosphorus".Journal of the American Chemical Society.36(7): 1344–1363.doi:10.1021/ja02184a002.ISSN0002-7863.
- ^Lange, Stefan; Schmidt, Peer; Nilges, Tom (2007). "Au3SnP7@Black Phosphorus: An Easy Access to Black Phosphorus".Inorganic Chemistry.46(10): 4028–35.doi:10.1021/ic062192q.PMID17439206.
- ^Vaghasiya, Jayraj V.; Mayorga–Martinez, Carmen C.; Vyskočil, Jan; Pumera, Martin (2023-01-03)."Black phosphorous-based human-machine communication interface".Nature Communications.14(1): 2.Bibcode:2023NatCo..14....2V.doi:10.1038/s41467-022-34482-4.ISSN2041-1723.PMC9810665.PMID36596775.
- ^Chemistry, University of; Prague, Technology."Black phosphorus–based human–machine communication interface: A breakthrough in assistive technology".techxplore.Retrieved2023-06-16.
- ^"Black Phosphorus Powder and Crystals".Ossila.Retrieved2019-08-23.
- ^Zhang, Yuanbo; Chen, Xian Hui; Feng, Donglai; Wu, Hua; Ou, Xuedong; Ge, Qingqin; Ye, Guo Jun; Yu, Yijun; Li, Likai (May 2014). "Black phosphorus field-effect transistors".Nature Nanotechnology.9(5): 372–377.arXiv:1401.4117.Bibcode:2014NatNa...9..372L.doi:10.1038/nnano.2014.35.ISSN1748-3395.PMID24584274.S2CID17218693.
- ^Wang, J.; Rousseau, A.; Yang, M.; Low, T.; Francoeur, S.; Kéna-Cohen, S. (2020). "Mid-infrared Polarized Emission from Black Phosphorus Light-Emitting Diodes".Nano Letters.20(5): 3651–3655.arXiv:1911.09184.Bibcode:2020NanoL..20.3651W.doi:10.1021/acs.nanolett.0c00581.PMID32286837.S2CID208202133.
- ^Smith, B.; Vermeersch, B.; Carrete, J.; Ou, E.; Kim, J.; Li, S. (2017)."Temperature and Thickness Dependences of the Anisotropic In-Plane Thermal Conductivity of Black Phosphorus".Adv Mater.29(5): 1603756.Bibcode:2017AdM....2903756S.doi:10.1002/adma.201603756.OSTI1533031.PMID27882620.S2CID5479539.
- ^Liu, Xiaolong D.; Wood, Joshua D.; Chen, Kan-Sheng; Cho, EunKyung; Hersam, Mark C. (9 February 2015). "In Situ Thermal Decomposition of Exfoliated Two-Dimensional Black Phosphorus".Journal of Physical Chemistry Letters.6(5): 773–778.arXiv:1502.02644.doi:10.1021/acs.jpclett.5b00043.PMID26262651.S2CID24648672.
- ^Wood, Joshua D.; Wells, Spencer A.; Jariwala, Deep; Chen, Kan-Sheng; Cho, EunKyung; Sangwan, Vinod K.; Liu, Xiaolong; Lauhon, Lincoln J.; Marks, Tobin J.; Hersam, Mark C. (7 November 2014). "Effective Passivation of Exfoliated Black Phosphorus Transistors against Ambient Degradation".Nano Letters.14(12): 6964–6970.arXiv:1411.2055.Bibcode:2014NanoL..14.6964W.doi:10.1021/nl5032293.PMID25380142.S2CID22128620.
- ^Wu, Ryan J.; Topsakal, Mehmet; Low, Tony; Robbins, Matthew C.; Haratipour, Nazila;Jeong, Jong Seok;Wentzcovitch, Renata M.; Koester, Steven J.; Mkhoyan, K. Andre (2015-11-01). "Atomic and electronic structure of exfoliated black phosphorus".Journal of Vacuum Science & Technology A.33(6): 060604.Bibcode:2015JVSTA..33f0604W.doi:10.1116/1.4926753.ISSN0734-2101.
- ^Zheng, Weiran; Lee, Jeongyeon; Gao, Zhi-Wen; Li, Yong; Lin, Shenghuang; Lau, Shu Ping; Lee, Lawrence Yoon Suk (30 June 2020). "Laser-Assisted Ultrafast Exfoliation of Black Phosphorus in Liquid with Tunable Thickness for Li-Ion Batteries".Advanced Energy Materials.10(31): 1903490.doi:10.1002/aenm.201903490.hdl:10397/100139.S2CID225707528.
- ^Karttunen, Antti J.; Linnolahti, Mikko; Pakkanen, Tapani A. (15 June 2007). "Icosahedral and Ring-Shaped Allotropes of Phosphorus".Chemistry – A European Journal.13(18): 5232–5237.doi:10.1002/chem.200601572.PMID17373003.
- ^Zhang, Jinying; Zhao, Dan; Xiao, Dingbin; Ma, Chuansheng; Du, Hongchu; Li, Xin; Zhang, Lihui; Huang, Jialiang; Huang, Hongyang; Jia, Chun-Lin; Tománek, David; Niu, Chunming (6 February 2017). "Assembly of Ring-Shaped Phosphorus within Carbon Nanotube Nanoreactors".Angewandte Chemie International Edition.56(7): 1850–1854.doi:10.1002/anie.201611740.PMID28074606.
- ^Zhang, Jia Lin; Zhao, Songtao (30 June 2016). "Epitaxial Growth of Single Layer Blue Phosphorus: A New Phase of Two-Dimensional Phosphorus".Nano Letters.16(8): 4903–4908.Bibcode:2016NanoL..16.4903Z.doi:10.1021/acs.nanolett.6b01459.PMID27359041.
- ^Piro, Na; Figueroa, Js; Mckellar, Jt; Cummins, Cc (2006). "Triple-bond reactivity of diphosphorus molecules".Science.313(5791): 1276–9.Bibcode:2006Sci...313.1276P.doi:10.1126/science.1129630.PMID16946068.S2CID27740669.
- ^abPfitzner, A; Bräu, Mf; Zweck, J; Brunklaus, G; Eckert, H (Aug 2004)."Phosphorus nanorods – two allotropic modifications of a long-known element".Angewandte Chemie International Edition in English.43(32): 4228–31.doi:10.1002/anie.200460244.PMID15307095.
- ^A. Holleman; N. Wiberg (1985). "XV 2.1.3".Lehrbuch der Anorganischen Chemie(33 ed.). de Gruyter.ISBN978-3-11-012641-9.
- ^Berger, L. I. (1996).Semiconductor materials.CRC Press. p.84.ISBN978-0-8493-8912-2.
External links[edit]
- White phosphorus
- White PhophorusatThe Periodic Table of Videos(University of Nottingham)
- More about White Phosphorus (and phosphorus pentoxide)atThe Periodic Table of Videos(University of Nottingham)
- The Chemistry of Phosphorusat Chemistry LibreTexts.

