Xylitol
| |
 Xylitol crystals
| |
| Names | |
|---|---|
| Pronunciation | /ˈzaɪlɪtɒl/ |
| IUPAC name
meso-Xylitol
| |
| Systematic IUPAC name
(2R,3R,4S)-Pentane-1,2,3,4,5-pentol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.626 |
| E number | E967(glazing agents,...) |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C5H12O5 | |
| Molar mass | 152.146g·mol−1 |
| Density | 1.52 g/cm3 |
| Melting point | 92 to 96 °C (198 to 205 °F; 365 to 369 K) |
| Boiling point | 345.39 °C (653.70 °F; 618.54 K) Predicted value using Adapted Stein & Brown method[2] |
| ~100 g/L | |
| Hazards | |
| NFPA 704(fire diamond) | |
| Related compounds | |
Relatedalkanes
|
Pentane |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
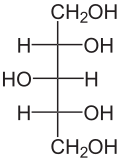
Xylitolis achemical compoundwith the formulaC
5H
12O
5,or HO(CH2)(CHOH)3(CH2)OH; specifically, one particularstereoisomerwith that structural formula. It is a colorless or whitecrystallinesolid that is freely soluble in water. It is classified as apolyalcoholand asugar alcohol,specifically analditol.The name derives fromAncient Greek:ξύλον,xyl[on]'wood', with the suffix-itolused to denote it being a sugar alcohol.
Xylitol is used as afood additiveandsugar substitute.ItsEuropean Union code numberisE967.[3]Replacingsugarwith xylitol in food products may promote better dental health, but evidence is lacking on whether xylitol itself preventsdental cavities.[4]
History
[edit]Emil Fischer,a German chemistry professor, and his assistant Rudolf Stahel isolated a new compound frombeechwood chips in September 1890 and named itXylit,the German word for xylitol. The following year, the French chemist M. G. Bertrand isolated xylitol syrup by processing wheat and oat straw.[5]Sugar rationing duringWorld War IIled to an interest insugar substitutes.Interest in xylitol and other polyols became intense, leading to theircharacterizationand manufacturing methods.[6][7]
Structure, production, commerce
[edit]Xylitol is one of three 5-carbon sugar alcohols. The others arearabitolandribitol.These three compounds differ in the stereochemistry of the three secondary alcohol groups.
Xylitol occurs naturally in small amounts in plums, strawberries, cauliflower, and pumpkin; humans and many other animals make trace amounts duringmetabolismofcarbohydrates.[6]Unlike most sugar alcohols, xylitol isachiral.[8]Most otherisomersof pentane-1,2,3,4,5-pentol are chiral, but xylitol has a plane of symmetry.
Industrial production starts withlignocellulosic biomassfrom whichxylanis extracted; raw biomass materials includehardwoods,softwoods,and agricultural waste from processing maize, wheat, or rice. The mixture is hydrolyzed with acid to givexylose.The xylose is purified bychromatography.Purifiedxyloseis catalyticallyhydrogenatedinto xylitol using aRaney nickelcatalyst.[9]The conversion changes the sugar (xylose, analdehyde) into theprimary alcohol,xylitol.[6]
Xylitol can also be obtained byindustrial fermentation,but this methodology are not as economical as the acid hydrolysis/chromatography route described above. Fermentation is effected by bacteria, fungi, or yeast, especiallyCandida tropicalis.[6][10]According to the USDepartment of Energy,xylitol production byfermentationfrom discardedbiomassis one of the most valuablerenewable chemicalsfor commerce, forecast to be a US $1.4 billion industry by 2025.[11]
Uses
[edit]Xylitol is used as asugar substitutein such manufactured products asdrugs,dietary supplements,confections,toothpaste,andchewing gum,but is not a common household sweetener.[4][12]Xylitol has negligible effects onblood sugarbecause its assimilation and metabolism are independent ofinsulin.[12]It is approved as a food additive in the United States[13]and elsewhere. Xylitol is often marketed as "birch sugar".
Xylitol is also found as an additive tosalinesolution fornasal irrigationand has been reported to be effective in improving symptoms of chronicsinusitis.[14]
Xylitol can also be incorporated into fabrics to produce a cooling fabric. When moisture, such as sweat, comes into contact with the xylitol embedded in the fabric, it produces a cooling sensation.[15]
Food properties
[edit]Nutrition, taste, and cooking
[edit]Humans absorb xylitol more slowly than sucrose, and xylitol supplies 40% fewer calories than an equal mass of sucrose.[12]
Xylitol has about the same sweetness assucrose,[12]but is sweeter than similar compounds likesorbitolandmannitol.[6]
Xylitol is stable enough to be used in baking,[16]but because xylitol and other polyols are more heat-stable, they do notcarameliseas sugars do. When used in foods, they lower the freezing point of the mixture.[17]
Food risks
[edit]No serious health risk exists in most humans for normal levels of consumption. TheEuropean Food Safety Authorityhas not set a limit on daily intake of xylitol. Due to the adverselaxativeeffect that all polyols have on the digestive system in high doses, xylitol is banned from soft drinks in theEuropean Union.Similarly, due to a 1985 report by the E.U.Scientific Committee on Foodwhich states that "ingesting 50 g a day of xylitol can causediarrhea",tabletop sweeteners (as well as other products containing xylitol) are required to display the warning" Excessive consumption may induce laxative effects ".[18]
Metabolism
[edit]Xylitol has 2.4 kilocalories offood energyper gram of xylitol (10 kilojoules per gram) according toU.S.andE.U.food-labeling regulations.[19][3]The real value can vary, depending on metabolic factors.[20]
Primarily, the liver metabolizes absorbed xylitol. The main metabolic route in humans occurs incytoplasm,via nonspecificNAD-dependent dehydrogenase (polyol dehydrogenase), which transforms xylitol toD-xylulose.Specificxylulokinasephosphorylates it toD-xylulose-5-phosphate.This then goes topentose phosphate pathwayfor further processing.[20]
About 50% of eaten xylitol is absorbed via the intestines. Of the remaining 50% that is not absorbed by the intestines, in humans, 50–75% of the xylitol remaining in the gut is fermented bygut bacteriainto short-chain organic acids and gases, which may produceflatulence.The remnant unabsorbed xylitol that escapes fermentation is excreted unchanged, mostly in feces; less than 2 g of xylitol out of every 100 g ingested is excreted via urine.[20]
Xylitol ingestion also increasesmotilinsecretion, which may be related to xylitol's ability to cause diarrhea.[21]The less-digestible but fermentable nature of xylitol also contributes to constipation relieving effects.[22]
Health effects
[edit]Dental care
[edit]A 2015Cochrane reviewof ten studies between 1991 and 2014 suggested a positive effect in reducingtooth decayof xylitol-containingfluoridetoothpastes when compared to fluoride-only toothpaste, but there was insufficient evidence to determine whether other xylitol-containing products can prevent tooth decay in infants, children or adults.[23]Subsequent reviews support the belief that xylitol can suppress the growth of pathogenicStreptococcusin the mouth, thereby reducing dental caries andgingivitis,although there is concern that swallowed xylitol may causeintestinal dysbiosis.[24][25][26]A 2022 review suggested that xylitol-containingchewing gumdecreases plaque, but not xylitol-containing candy.[27]
Earache
[edit]In 2011EFSA"concluded that there was not enough evidence to support" the claim that xylitol-sweetened gum could prevent middle-ear infections, also known asacute otitis media(AOM).[18][28]A 2016 review indicated that xylitol in chewing gum or a syrup may have a moderate effect in preventing AOM in healthy children.[29]It may be an alternative to conventional therapies (such asantibiotics) to lower risk of earache in healthy children – reducing risk of occurrence by 25%[30]– although there is no definitive proof that it could be used as a therapy for earache.[29]
Diabetes
[edit]In 2011, EFSA approved a marketing claim that foods or beverages containing xylitol or similar sugar replacers cause lower blood glucose and lowerinsulinresponses compared to sugar-containing foods or drinks.[16][31]Xylitol products are used assucrosesubstitutes for weight control,[16][22]as xylitol has 40% fewer calories than sucrose (2.4 kcal/g compared to 4.0 kcal/g for sucrose).[16][32]Theglycemic index(GI) of xylitol is only 7% of the GI forglucose.[33]
Adverse effects
[edit]Humans
[edit]When ingested at high doses, xylitol and other polyols may causegastrointestinaldiscomfort, includingflatulence,diarrhea,andirritable bowel syndrome(seeMetabolismabove); some people experience the adverse effects at lower doses.[18][34]Xylitol has a lowerlaxationthreshold than somesugar alcoholsbut is more easily tolerated thanmannitolandsorbitol.[35]
Increased xylitol consumption can increase oxalate, calcium, and phosphate excretion to urine (termedoxaluria,calciuria,andphosphaturia,respectively). These are known risk factors forkidney stone disease,but despite that, xylitol has not been linked to kidney disease in humans.[36]
Dogs and other animals
[edit]Xylitol is poisonous todogs.[37]Ingesting 100 milligrams of xylitol per kilogram of body weight (mg/kg bw) causes dogs to experience a dose-dependentinsulinrelease; depending on the dose it can result in life-threateninghypoglycemia.Hypoglycemic symptoms of xylitol toxicity may arise as quickly as 30 to 60 minutes after ingestion. Vomiting is a common first symptom, which can be followed by tiredness andataxia.At doses above 500 mg/kg bw,liver failureis likely and may result incoagulopathieslikedisseminated intravascular coagulation.[38]
Xylitol is safe forrhesus macaques,horses,andrats.[38]
A 2018 study suggests that xylitol is safe forcatsin doses of up to 1000 mg/kg; however, this study was performed on only 6 cats and should not be considered definitive.[39]
See also
[edit]References
[edit]- ^Safety data sheetforxylitolArchived3 March 2016 at theWayback MachinefromFisher Scientific.Retrieved 2014-11-02.
- ^ "Xylitol".Chemspider.Chemical Structure.Retrieved13 May2015.
- ^ab "Food legislation".polyols-eu.org.European Association of Polyol Producers. 22 March 2017.Retrieved7 February2019.
- ^ab
Riley, P.; Moore, D.; Ahmed, F.; Sharif, M.O.; Worthington, H.V. (26 March 2015)."Xylitol-containing products for preventing dental caries in children and adults".The Cochrane Database of Systematic Reviews.2015(3): CD010743.doi:10.1002/14651858.CD010743.pub2.PMC9345289.PMID25809586.
Riley, P.; Moore, D.; Ahmed, F.; Sharif, M. O.; Worthington, H. V. (2015)."Can xylitol – used in products like sweets, candy, chewing gum, and toothpaste – help prevent tooth decay in children and adults?".The Cochrane Database of Systematic Reviews.Lay summary.2015(3): CD010743.doi:10.1002/14651858.CD010743.pub2.PMC9345289.PMID25809586.
- ^Mäkinen KK (June 2000)."The rocky road of xylitol to its clinical application".Journal of Dental Research.79(6): 1352–5.doi:10.1177/00220345000790060101.PMID10890712.S2CID31432699.
- ^abcde Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. (2015). "Xylitol: A review on bio-production, application, health benefits, and related safety issues".Critical Reviews in Food Science and Nutrition.55(11): 1514–1528.doi:10.1080/10408398.2012.702288.PMID24915309.S2CID20359589.
- ^ Hicks, Jesse (Spring 2010)."The pursuit of sweet".Science History.Science History Institute.
- ^ Wrolstad, Ronald E. (2012).Food Carbohydrate Chemistry.John Wiley & Sons. p. 176.ISBN9780813826653.Retrieved20 October2012– via Google Books.
- ^Schiweck, Hubert; Bär, Albert; Vogel, Roland; Schwarz, Eugen; Kunz, Markwart; Dusautois, Cécile; Clement, Alexandre; Lefranc, Caterine; Lüssem, Bernd; Moser, Matthias; Peters, Siegfried (2012). "Sugar Alcohols".Ullmann's Encyclopedia of Industrial Chemistry.doi:10.1002/14356007.a25_413.pub3.ISBN9783527303854.
- ^ Jain, H.; Mulay, S. (March 2014). "A review on different modes and methods for yielding a pentose sugar: Xylitol".International Journal of Food Sciences and Nutrition.65(2): 135–143.doi:10.3109/09637486.2013.845651.PMID24160912.S2CID39929588.
- ^ Felipe Hernández-Pérez, Andrés; de Arruda, Priscila Vaz; Sene, Luciane; da Silva, Silvio Silvério; Kumar Chandel, Anuj; de Almeida Felipe, Maria das Graças (16 July 2019). "Xylitol bioproduction: State-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries".Critical Reviews in Biotechnology.39(7): 924–943.doi:10.1080/07388551.2019.1640658.ISSN0738-8551.PMID31311338.S2CID197421362.
- ^abcd "Xylitol".Drugs.2018.Retrieved12 October2018.
- ^ "Xylitol".United StatesCode of Federal Regulations.Food Additives Permitted for Direct Addition to Food for Human Consumption, Special Dietary and Nutritional Additives. U.S.Food and Drug Administration.1 April 2012. CFR Title 21, Part 172, Section 172.395.
- ^ Weissman, Joshua D.; Fernandez, Francisca; Hwang, Peter H. (November 2011)."Xylitol nasal irrigation in the management of chronic rhinosinusitis: A pilot study".The Laryngoscope.121(11): 2468–2472.doi:10.1002/lary.22176.ISSN1531-4995.PMID21994147.S2CID36572019.
- ^Peng, Yucan; Cui, Yi (15 April 2020)."Advanced Textiles for Personal Thermal Management and Energy".Joule.4(4): 724–742.Bibcode:2020Joule...4..724P.doi:10.1016/j.joule.2020.02.011.ISSN2542-4351.
- ^abcd "Xylitol".Diabetes.co.uk.Retrieved28 October2018.
- ^Burgos, Karen; Subramaniam, Persis; Arthur, Jennifer (21 November 2016)."Reformulation guide for small to medium sized companies"(PDF).Leatherhead Food Research. Archived fromthe original(PDF)on 27 September 2020.Retrieved28 October2018– via The Food and Drink Federation.
- ^abc "Is xylitol good for your teeth?".Live well: Eat well. U.K.National Health Service.13 April 2016.Retrieved28 October2018.
- ^ "Chapter 3: Energy Conversion Factors".Calculation of the Energy Content of Foods. Food and Agriculture Organization (Report). The United Nations.Retrieved30 March2017.
- ^abc Livesey, G. (2003)."Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties".Nutrition Research Reviews.16(2): 163–191.doi:10.1079/NRR200371.ISSN1475-2700.PMID19087388.
- ^ Wölnerhanssen, B. K.; Meyer-Gerspach, A. C.; Beglinger, C.; Islam, M. S. (June 2019). "Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review".Critical Reviews in Food Science and Nutrition.60(12): 1986–1998.doi:10.1080/10408398.2019.1623757.PMID31204494.S2CID189944738.
- ^ab Salli, Krista; Lehtinen, Markus J.; Tiihonen, Kirsti; Ouwehand, Arthur C. (6 August 2019)."Xylitol's health benefits beyond dental health: A comprehensive review".Nutrients.11(8): 1813.doi:10.3390/nu11081813.ISSN2072-6643.PMC6723878.PMID31390800.
- ^Riley P, Moore D, Ahmed F, Worthington HV (2015)."Xylitol-containing products for preventing dental caries in children and adults".Cochrane Library#The_Cochrane Database of Systematic Reviews.2015(3): CD010743.doi:10.1002/14651858.CD010743.pub2.PMC9345289.PMID25809586.
- ^Benahmed AG, Gasmi A, Bjørklund G (2020). "Health benefits of xylitol".Applied Microbiology and Biotechnology.104(17): 225–7237.doi:10.1007/s00253-020-10708-7.PMID32638045.
- ^ALHumaid J, Bamashmous M (2022)."Meta-analysis on the Effectiveness of Xylitol in Caries Prevention".Journal of International Society of Preventive & Community Dentistry.12(2): 133–138.doi:10.4103/jispcd.JISPCD_164_21.PMC9022379.PMID35462747.
- ^Janket S, Benwait J, Meurman JH (2019). "Oral and Systemic Effects of Xylitol Consumption".Caries Research.53(5): 491–501.doi:10.1159/000499194.PMID31060040.
- ^Söderling, Eva; Pienihäkkinen, Kaisu (2022)."Effects of xylitol chewing gum and candies on the accumulation of dental plaque: a systematic review".Clinical Oral Investigations.26(1): 119–129.doi:10.1007/s00784-021-04225-8.ISSN1432-6981.PMC8791908.PMID34677696.
- ^ EFSA pannel (June 2011)."Scientific opinion on the substantiation of health claims related to sugar-free chewing gum sweetened with xylitol and plaque acid neutralisation (ID 485), maintenance of tooth mineralisation (ID 486, 562, 1181), reduction of dental plaque (ID 485, 3085)".EFSA Journal.9(6): 2266.doi:10.2903/j.efsa.2011.2266.
- ^ab Azarpazhooh, A.; Lawrence, H.P.; Shah, P.S. (3 August 2016)."Xylitol for preventing acute otitis media in children up to 12 years of age".The Cochrane Database of Systematic Reviews.2016(8): CD007095.doi:10.1002/14651858.CD007095.pub3.PMC8485974.PMID27486835.
- ^ Marom, Tal; Marchisio, Paola; Tamir, Sharon Ovnat; Torretta, Sara; Gavriel, Haim; Esposito, Susanna (12 February 2016)."Complementary and alternative medicine treatment options for otitis media".Medicine.95(6): e2695.doi:10.1097/MD.0000000000002695.ISSN0025-7974.PMC4753897.PMID26871802.
- ^ EFSA panel (April 2011)."Scientific opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation, and reduction of post-prandial glycaemic responses".EFSA Journal.9(4): 2076.doi:10.2903/j.efsa.2011.2076.
- ^ Tiefenbacher, Karl F. (2017). "Technology of Main Ingredients – Sweeteners and Lipids".Wafer and Waffle.Elsevier. pp. 123–225.doi:10.1016/b978-0-12-809438-9.00003-x.ISBN978-0-12-809438-9.
- ^ Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. (July 2002)."International table of glycemic index and glycemic load values: 2002".The American Journal of Clinical Nutrition.76(1): 5–56.doi:10.1093/ajcn/76.1.5.PMID12081815.
- ^ Mäkinen, Kauko (20 October 2016)."Gastrointestinal disturbances associated with the consumption of sugar alcohols with special consideration of xylitol: Scientific review and instructions for dentists and other health-care professionals".International Journal of Dentistry.2016:5967907.doi:10.1155/2016/5967907.PMC5093271.PMID27840639.
- ^ Sugar Alcohols(PDF)(Report). Canadian Diabetes Association. 1 May 2005. Archived fromthe original(PDF)on 25 April 2012.Retrieved14 March2012.
- ^ Janket, S.; Benwait, J.; Isaac, P.; Ackerson, L.K.; Meurman, J.H. (2019). "Oral and systemic effects of xylitol consumption".Caries Research.53(5): 491–501.doi:10.1159/000499194.hdl:10138/305074.PMID31060040.S2CID146811298.
- ^"Paws off xylitol; It's dangerous for dogs".US Food and Drug Administration. 7 July 2021.Retrieved9 September2021.
- ^ab Schmid, R. D.; Hovda, L. R. (2016)."Acute hepatic failure in a dog after xylitol ingestion".Journal of Medical Toxicology.12(2): 201–205.doi:10.1007/s13181-015-0531-7.PMC4880608.PMID26691320.
- ^ Jerzsele, A.; et al. (2018)."Effects of p.o. administered xylitol in cats".Journal of Veterinary Pharmacology and Therapeutics.41(3): 409–414.doi:10.1111/jvp.12479.PMID29430681.




