Zomebazam
Appearance
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
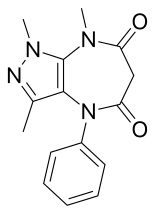
| Formula | C15H16N4O2 |
| Molar mass | 284.319g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zomebazam[1]produced byHoechstis a pyrazolodiazepinone derivative drug withanxiolyticproperties. It is structurally related torazobazamandzometapine.[2]
Synthesis
[edit]The catalytic hydrogenation ofN,2,5-trimethyl-4-phenyldiazenylpyrazol-3-amine[a](1) overRaney nickelgives 4-amino-1,3-dimethyl-5-methylaminopyrazole[b](2). Treatment with methyl malonyl chloride[c](3) gives 4-α-ethoxycarbonylacetylamino-1,3-dimethyl-5-methylaminopyrazole[d](4). Base-catalyzed lactamization gives (5). TheGoldberg reactioncompletes the synthesis of zomebazam (6).[3][4]

See also
[edit]References
[edit]- ^US 3558605,"4-Aryl-5,6,7,8-tetrahydropyrazolo(3,4-B)-(1,5)diazepine-1H,4H-5,7-diones and medicaments containing same"
- ^"Zomebazam".psychotropics.dk. 2003.Retrieved7 December2009.
- ^Renger B (1985). "Direkte N-Arylierung von Amiden: Eine Verbesserung der Goldberg-Reaktion".Synthesis.1985(9): 856–560.doi:10.1055/s-1985-31364.S2CID93397774.
- ^US 4302468,Rackur G, Hoffmann I, issued 1981, assigned to Hoechst Aktiengesellschaft
Notes
[edit]- ^CID:136203602
- ^CID:10219477
- ^CAS# [37517-81-0]
- ^CID:20561101
