Enzyme
Enzymesareproteinsthatcatalyze(i.e.,increase or decrease the ratesof)chemical reactions.[1][2]In enzymatic reactions, themoleculesat the beginning of the process are calledsubstrates,and they are converted into different molecules, called theproducts.Almost all processes in abiological cellneed enzymes to occur at significant rates. Since enzymes are selective for their substrates and speed up only a few reactions from among many possibilities, the set of enzymes made in a cell determines whichmetabolic pathwaysoccur in that cell.
Like all catalysts, enzymes work by lowering theactivation energy(Ea‡) for a reaction, thus dramatically increasing the rate of the reaction. As a result, products are formed faster and reactions reach their equilibrium state more rapidly. Most enzyme reaction rates are millions of times faster than those of comparable un-catalyzed reactions. As with all catalysts, enzymes are not consumed by the reactions they catalyze, nor do they alter theequilibriumof these reactions. However, enzymes do differ from most other catalysts by being much more specific. Enzymes are known to catalyze about 4,000 biochemical reactions.[3]A fewRNAmolecules calledribozymesalso catalyze reactions, with an important example being some parts of theribosome.[4][5]Synthetic molecules calledartificial enzymesalso display enzyme-like catalysis.[6]
Enzyme activity can be affected by other molecules.Inhibitorsare molecules that decrease enzyme activity;activatorsare molecules that increase activity. Manydrugsandpoisonsare enzyme inhibitors. Activity is also affected bytemperature,chemical environment (e.g.,pH), and theconcentrationof substrate. Some enzymes are used commercially, for example, in the synthesis ofantibiotics.In addition, some household products use enzymes to speed up biochemical reactions (e.g.,enzymes in biologicalwashing powdersbreak down protein orfatstains on clothes; enzymes inmeat tenderizersbreak down proteins into smaller molecules, making the meat easier to chew).
Etymology and history

As early as the late 18th and early 19th centuries, the digestion ofmeatby stomach secretions[7]and the conversion ofstarchtosugarsby plant extracts andsalivawere known. However, the mechanism by which this occurred had not been identified.[8]
In the 19th century, when studying thefermentationof sugar toalcoholbyyeast,Louis Pasteurcame to the conclusion that this fermentation was catalyzed by a vital force contained within the yeast cells called "ferments",which were thought to function only within living organisms. He wrote that" alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells. "[9]
In 1877, German physiologistWilhelm Kühne(1837–1900) first used the termenzyme,which comes fromGreekενζυμον,"in leaven", to describe this process.[10]The wordenzymewas used later to refer to nonliving substances such aspepsin,and the wordfermentwas used to refer to chemical activity produced by living organisms.
In 1897,Eduard Buchnersubmitted his first paper on the ability of yeast extracts that lacked any living yeast cells to ferment sugar. In a series of experiments at theUniversity of Berlin,he found that the sugar was fermented even when there were no living yeast cells in the mixture.[11]He named the enzyme that brought about the fermentation of sucrose "zymase".[12]In 1907, he received theNobel Prize in Chemistry"for his biochemical research and his discovery of cell-free fermentation". Following Buchner's example, enzymes are usually named according to the reaction they carry out. Typically, to generate the name of an enzyme, the suffix-aseis added to the name of itssubstrate(e.g.,lactaseis the enzyme that cleaveslactose) or the type of reaction (e.g.,DNA polymeraseforms DNA polymers).[13]
Having shown that enzymes could function outside a living cell, the next step was to determine their biochemical nature. Many early workers noted that enzymatic activity was associated with proteins, but several scientists (such as Nobel laureateRichard Willstätter) argued that proteins were merely carriers for the true enzymes and that proteinsper sewere incapable of catalysis. However, in 1926,James B. Sumnershowed that the enzymeureasewas a pure protein and crystallized it; Sumner did likewise for the enzymecatalasein 1937. The conclusion that pure proteins can be enzymes was definitively proved byNorthropandStanley,who worked on the digestive enzymes pepsin (1930), trypsin and chymotrypsin. These three scientists were awarded the 1946 Nobel Prize in Chemistry.[14]
This discovery that enzymes could be crystallized eventually allowed their structures to be solved byx-ray crystallography.This was first done forlysozyme,an enzyme found in tears, saliva andegg whitesthat digests the coating of some bacteria; the structure was solved by a group led byDavid Chilton Phillipsand published in 1965.[15]This high-resolution structure of lysozyme marked the beginning of the field ofstructural biologyand the effort to understand how enzymes work at an atomic level of detail.
Structures and mechanisms

Enzymes are generallyglobular proteinsand range from just 62 amino acid residues in size, for themonomerof4-oxalocrotonate tautomerase,[16]to over 2,500 residues in the animalfatty acid synthase.[17]A small number of RNA-based biological catalysts exist, with the most common being theribosome;these are referred to as either RNA-enzymes orribozymes.The activities of enzymes are determined by theirthree-dimensional structure.[18]However, although structure does determine function, predicting a novel enzyme's activity just from its structure is a very difficult problem that has not yet been solved.[19]
Most enzymes are much larger than the substrates they act on, and only a small portion of the enzyme (around 3–4amino acids) is directly involved in catalysis.[20]The region that contains these catalytic residues, binds the substrate, and then carries out the reaction is known as theactive site.Enzymes can also contain sites that bindcofactors,which are needed for catalysis. Some enzymes also have binding sites for small molecules, which are often direct orindirectproducts or substrates of the reaction catalyzed. This binding can serve to increase or decrease the enzyme's activity, providing a means forfeedbackregulation.
Like all proteins, enzymes are long, linear chains of amino acids thatfoldto produce athree-dimensional product.Each unique amino acid sequence produces a specific structure, which has unique properties. Individual protein chains may sometimes group together to form aprotein complex.Most enzymes can bedenatured—that is, unfolded and inactivated—by heating or chemical denaturants, which disrupt thethree-dimensional structureof the protein. Depending on the enzyme, denaturation may be reversible or irreversible.
Structures of enzymes in complex with substrates or substrate analogs during a reaction may be obtained usingTime resolved crystallographymethods.
Specificity
Enzymes are usually very specific as to which reactions they catalyze and thesubstratesthat are involved in these reactions. Complementary shape, charge andhydrophilic/hydrophobiccharacteristics of enzymes and substrates are responsible for this specificity. Enzymes can also show impressive levels ofstereospecificity,regioselectivityandchemoselectivity.[21]
Some of the enzymes showing the highest specificity and accuracy are involved in the copying andexpressionof thegenome.These enzymes have "proof-reading" mechanisms. Here, an enzyme such asDNA polymerasecatalyzes a reaction in a first step and then checks that the product is correct in a second step.[22]This two-step process results in average error rates of less than 1 error in 100 million reactions in high-fidelitymammalianpolymerases.[23]Similar proofreading mechanisms are also found inRNA polymerase,[24]aminoacyl tRNA synthetases[25]andribosomes.[26]
Some enzymes that producesecondary metabolitesare described as promiscuous, as they can act on a relatively broad range of different substrates. It has been suggested that this broad substrate specificity is important for the evolution of new biosynthetic pathways.[27]
"Lock and key" model
Enzymes are very specific, and it was suggested by theNobel laureateorganic chemistEmil Fischerin 1894 that this was because both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.[28]This is often referred to as "the lock and key" model. However, while this model explains enzyme specificity, it fails to explain the stabilization of the transition state that enzymes achieve.

In 1958,Daniel Koshlandsuggested a modification to the lock and key model: since enzymes are rather flexible structures, the active site is continually reshaped by interactions with the substrate as the substrate interacts with the enzyme.[29]As a result, the substrate does not simply bind to a rigid active site; the amino acidside chainswhich make up the active site are molded into the precise positions that enable the enzyme to perform its catalytic function. In some cases, such as glycosidases, the substrate molecule also changes shape slightly as it enters the active site.[30]The active site continues to change until the substrate is completely bound, at which point the final shape and charge is determined.[31] Induced fit may enhance the fidelity of molecular recognition in the presence of competition and noise via theconformational proofreadingmechanism .[32]
Mechanisms
Enzymes can act in several ways, all of which lower ΔG‡:[33]
- Lowering theactivation energyby creating an environment in which the transition state is stabilized (e.g. straining the shape of a substrate—by binding the transition-state conformation of the substrate/product molecules, the enzyme distorts the bound substrate(s) into their transition state form, thereby reducing the amount of energy required to complete the transition).
- Lowering the energy of the transition state, but without distorting the substrate, by creating an environment with the opposite charge distribution to that of the transition state.
- Providing an alternative pathway. For example, temporarily reacting with the substrate to form an intermediate ES complex, which would be impossible in the absence of the enzyme.
- Reducing the reaction entropy change by bringing substrates together in the correct orientation to react. Considering ΔH‡alone overlooks this effect.
- Increases in temperatures speed up reactions. Thus, temperature increases help the enzyme function and develop the end product even faster. However, if heated too much, the enzyme’s shape deteriorates and the enzyme becomes denatured. Some enzymes like thermolabile enzymes work best at low temperatures.
Interestingly, thisentropiceffect involves destabilization of the ground state,[34]and its contribution to catalysis is relatively small.[35]
Transition State Stabilization
The understanding of the origin of the reduction of ΔG‡requires one to find out how the enzymes can stabilize its transition state more than the transition state of the uncatalyzed reaction. Apparently, the most effective way for reaching large stabilization is the use of electrostatic effects, in particular, by having a relatively fixed polar environment that is oriented toward the charge distribution of the transition state.[36]Such an environment does not exist in the uncatalyzed reaction in water.
Dynamics and function
The internal dynamics of enzymes is linked to their mechanism of catalysis.[37][38][39] Internal dynamics are the movement of parts of the enzyme's structure, such as individual amino acid residues, a group of amino acids, or even an entireprotein domain.These movements occur at various time-scales ranging fromfemtosecondsto seconds. Networks of protein residues throughout an enzyme's structure can contribute to catalysis through dynamic motions.[40][41][42][43]Protein motions are vital to many enzymes, but whether small and fast vibrations, or larger and slower conformational movements are more important depends on the type of reaction involved. However, although these movements are important in binding and releasing substrates and products, it is not clear if protein movements help to accelerate the chemical steps in enzymatic reactions.[44]These new insights also have implications in understanding allosteric effects and developing new drugs.
Allosteric modulation

Allosteric sites are sites on the enzyme that bind to molecules in the cellular environment. The sites form weak, noncovalent bonds with these molecules, causing a change in the conformation of the enzyme. This change in conformation translates to the active site, which then affects the reaction rate of the enzyme.[45]Allosteric interactions can both inhibit and activate enzymes and are a common way that enzymes are controlled in the body.[46]
Cofactors and coenzymes
Cofactors
Some enzymes do not need any additional components to show full activity. However, others require non-protein molecules called cofactors to be bound for activity.[47]Cofactors can be eitherinorganic(e.g.,metal ionsandiron-sulfur clusters) ororganic compounds(e.g.,flavinandheme). Organic cofactors can be eitherprosthetic groups,which are tightly bound to an enzyme, orcoenzymes,which are released from the enzyme's active site during the reaction. Coenzymes includeNADH,NADPHandadenosine triphosphate.These molecules transfer chemical groups between enzymes.[48]
An example of an enzyme that contains a cofactor iscarbonic anhydrase,and is shown in theribbon diagramabove with a zinc cofactor bound as part of its active site.[49]These tightly bound molecules are usually found in the active site and are involved in catalysis. For example, flavin and heme cofactors are often involved inredoxreactions.
Enzymes that require a cofactor but do not have one bound are calledapoenzymesorapoproteins.An apoenzyme together with its cofactor(s) is called aholoenzyme(this is the active form). Most cofactors are not covalently attached to an enzyme, but are very tightly bound. However, organic prosthetic groups can be covalently bound (e.g.,thiamine pyrophosphatein the enzymepyruvate dehydrogenase). The term "holoenzyme" can also be applied to enzymes that contain multiple protein subunits, such as theDNA polymerases;here the holoenzyme is the complete complex containing all the subunits needed for activity.
Coenzymes
Coenzymes are small organic molecules that can be loosely bind to enzyme or tightly binds to enzyme. In case, coenzyme binds tighly to enzyme is called allosteric group. Coenzymes are transport chemical groups from one enzyme to another.[50]Some of these chemicals such asriboflavin,thiamineandfolic acidarevitamins(compounds which cannot be synthesized by the body and must be acquired from the diet). The chemical groups carried include thehydrideion (H-) carried byNAD or NADP+,the phosphate group carried byadenosine triphosphate,the acetyl group carried bycoenzyme A,formyl, methenyl or methyl groups carried byfolic acidand the methyl group carried byS-adenosylmethionine.
Since coenzymes are chemically changed as a consequence of enzyme action, it is useful to consider coenzymes to be a special class of substrates, or second substrates, which are common to many different enzymes. For example, about 700 enzymes are known to use the coenzyme NADH.[51]
Coenzymes are usually continuously regenerated and their concentrations maintained at a steady level inside the cell: for example, NADPH is regenerated through thepentose phosphate pathwayandS-adenosylmethionine bymethionine adenosyltransferase.This continuous regeneration means that even small amounts of coenzymes are used very intensively. For example, the human body turns over its own weight in ATP each day.[52]
Thermodynamics

As all catalysts, enzymes do not alter the position of the chemical equilibrium of the reaction. Usually, in the presence of an enzyme, the reaction runs in the same direction as it would without the enzyme, just more quickly. However, in the absence of the enzyme, other possible uncatalyzed, "spontaneous" reactions might lead to different products, because in those conditions this different product is formed faster.
Furthermore, enzymes can couple two or more reactions, so that a thermodynamically favorable reaction can be used to "drive" a thermodynamically unfavorable one. For example, the hydrolysis ofATPis often used to drive other chemical reactions.[53]
Enzymes catalyze the forward and backward reactions equally. They do not alter the equilibrium itself, but only the speed at which it is reached. For example,carbonic anhydrasecatalyzes its reaction in either direction depending on the concentration of its reactants.
Nevertheless, if the equilibrium is greatly displaced in one direction, that is, in a veryexergonicreaction, the reaction iseffectivelyirreversible. Under these conditions the enzyme will, in fact, only catalyze the reaction in the thermodynamically allowed direction.
Kinetics

Enzyme kinetics is the investigation of how enzymes bind substrates and turn them into products. The rate data used in kinetic analyses are obtained fromenzyme assays.
In 1902 Victor Henri[54]proposed a quantitative theory of enzyme kinetics, but his experimental data were not useful because the significance of the hydrogen ion concentration was not yet appreciated. AfterPeter Lauritz Sørensenhad defined the logarithmic pH-scale and introduced the concept of buffering in 1909[55]the German chemistLeonor Michaelisand his Canadian postdocMaud Leonora Mentenrepeated Henri's experiments and confirmed his equation which is referred to asHenri-Michaelis-Menten kinetics(sometimes alsoMichaelis-Menten kinetics).[56]Their work was further developed byG. E. BriggsandJ. B. S. Haldane,who derived kinetic equations that are still widely used today.[57]
The major contribution of Henri was to think of enzyme reactions in two stages. In the first, the substrate binds reversibly to the enzyme, forming the enzyme-substrate complex. This is sometimes called the Michaelis complex. The enzyme then catalyzes the chemical step in the reaction and releases the product.
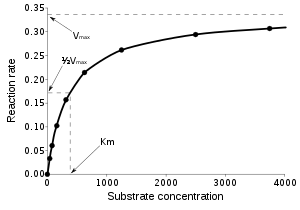
Enzymes can catalyze up to several million reactions per second. For example, the uncatalyzed decarboxylation oforotidine 5'-monophosphatehas a half life of 78 million years. However, when the enzymeorotidine 5'-phosphate decarboxylaseis added, the same process takes just 25 milliseconds.[58]Enzyme rates depend on solution conditions and substrate concentration. Conditions that denature the protein abolish enzyme activity, such as high temperatures, extremes of pH or high salt concentrations, while raising substrate concentration tends to increase activity. To find the maximum speed of an enzymatic reaction, the substrate concentration is increased until a constant rate of product formation is seen. This is shown in the saturation curve on the right. Saturation happens because, as substrate concentration increases, more and more of the free enzyme is converted into the substrate-bound ES form. At the maximum velocity (Vmax) of the enzyme, all the enzyme active sites are bound to substrate, and the amount of ES complex is the same as the total amount of enzyme. However,Vmaxis only one kinetic constant of enzymes. The amount of substrate needed to achieve a given rate of reaction is also important. This is given by theMichaelis-Menten constant(Km), which is the substrate concentration required for an enzyme to reach one-half its maximum velocity. Each enzyme has a characteristicKmfor a given substrate, and this can show how tight the binding of the substrate is to the enzyme. Another useful constant iskcat,which is the number of substrate molecules handled by one active site per second.
The efficiency of an enzyme can be expressed in terms ofkcat/Km.This is also called the specificity constant and incorporates therate constantsfor all steps in the reaction. Because the specificity constant reflects both affinity and catalytic ability, it is useful for comparing different enzymes against each other, or the same enzyme with different substrates. The theoretical maximum for the specificity constant is called the diffusion limit and is about 108to 109(M−1s−1). At this point every collision of the enzyme with its substrate will result in catalysis, and the rate of product formation is not limited by the reaction rate but by the diffusion rate. Enzymes with this property are calledcatalytically perfectorkinetically perfect.Example of such enzymes aretriose-phosphate isomerase,carbonic anhydrase,acetylcholinesterase,catalase,fumarase, β-lactamase, andsuperoxide dismutase.
Michaelis-Menten kinetics relies on thelaw of mass action,which is derived from the assumptions of freediffusionand thermodynamically driven random collision. However, many biochemical or cellular processes deviate significantly from these conditions, because ofmacromolecular crowding,phase-separation of the enzyme/substrate/product, or one or two-dimensional molecular movement.[59]In these situations, afractalMichaelis-Menten kineticsmay be applied.[60][61][62][63]
Some enzymes operate with kinetics which are faster than diffusion rates, which would seem to be impossible. Several mechanisms have been invoked to explain this phenomenon. Some proteins are believed to accelerate catalysis by drawing their substrate in and pre-orienting them by using dipolar electric fields. Other models invoke a quantum-mechanicaltunnelingexplanation, whereby a proton or an electron can tunnel through activation barriers, although for proton tunneling this model remains somewhat controversial.[64][65]Quantum tunneling for protons has been observed intryptamine.[66]This suggests that enzyme catalysis may be more accurately characterized as "through the barrier" rather than the traditional model, which requires substrates to go "over" a lowered energy barrier.
Inhibition
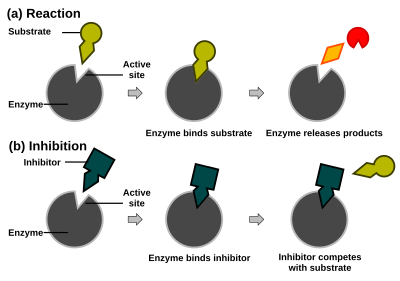

Enzyme reaction rates can be decreased by various types ofenzyme inhibitors.
- Competitive inhibition
In competitive inhibition, the inhibitor and substrate compete for the enzyme (i.e., they can not bind at the same time).[68]Often competitive inhibitors strongly resemble the real substrate of the enzyme. For example,methotrexateis a competitive inhibitor of the enzymedihydrofolate reductase,which catalyzes the reduction ofdihydrofolatetotetrahydrofolate.The similarity between the structures of folic acid and this drug are shown in the figure to therightbottom. Note that binding of the inhibitor neednotbe to the substrate binding site (as frequently stated), if binding of the inhibitor changes the conformation of the enzyme to prevent substrate binding andvice versa.In competitive inhibition the maximal velocity of the reaction is not changed, but higher substrate concentrations are required to reach a given velocity, increasing the apparent Km.
- Uncompetitive inhibition
In uncompetitive inhibition the inhibitor can not bind to the free enzyme, but only to the ES-complex. The EIS-complex thus formed is enzymatically inactive. This type of inhibition is rare, but may occur in multimeric enzymes.
- Non-competitive inhibition
Non-competitive inhibitors can bind to the enzyme at the binding site at the same time as the substrate,but not to the active site. Both the EI and EIS complexes are enzymatically inactive. Because the inhibitor can not be driven from the enzyme by higher substrate concentration (in contrast to competitive inhibition), the apparent Vmaxchanges. But because the substrate can still bind to the enzyme, the Kmstays the same.
- Mixed inhibition
This type of inhibition resembles the non-competitive, except that the EIS-complex has residual enzymatic activity.This type of inhibitor does not follow Michaelis-Menten equation.
In many organisms inhibitors may act as part of afeedbackmechanism. If an enzyme produces too much of one substance in the organism, that substance may act as an inhibitor for the enzyme at the beginning of the pathway that produces it, causing production of the substance to slow down or stop when there is sufficient amount. This is a form ofnegative feedback.Enzymes which are subject to this form of regulation are often multimeric and have allosteric binding sites for regulatory substances. Their substrate/velocity plots are not hyperbolar, but sigmoidal (S-shaped).
Irreversible inhibitorsreact with the enzyme and form acovalentadduct with the protein. The inactivation is irreversible. These compounds includeeflornithinea drug used to treat the parasitic diseasesleeping sickness.[69]PenicillinandAspirinalso act in this manner. With these drugs, the compound is bound in the active site and the enzyme then converts the inhibitor into an activated form that reacts irreversibly with one or more amino acid residues.
- Uses of inhibitors
Since inhibitors modulate the function of enzymes they are often used as drugs. An common example of an inhibitor that is used as a drug isaspirin,which inhibits theCOX-1andCOX-2enzymes that produce theinflammationmessengerprostaglandin,thus suppressing pain and inflammation. However, other enzyme inhibitors are poisons. For example, the poisoncyanideis an irreversible enzyme inhibitor that combines with the copper and iron in the active site of the enzymecytochrome c oxidaseand blockscellular respiration.[70]
Biological function
Enzymes serve a wide variety offunctionsinside living organisms. They are indispensable forsignal transductionand cell regulation, often viakinasesandphosphatases.[71]They also generate movement, withmyosinhydrolysing ATP to generatemuscle contractionand also moving cargo around the cell as part of thecytoskeleton.[72]Other ATPases in the cell membrane areion pumpsinvolved inactive transport.Enzymes are also involved in more exotic functions, such asluciferasegenerating light infireflies.[73]Virusescan also contain enzymes for infecting cells, such as theHIV integraseandreverse transcriptase,or for viral release from cells, like theinfluenzavirusneuraminidase.
An important function of enzymes is in thedigestive systemsof animals. Enzymes such asamylasesandproteasesbreak down large molecules (starchorproteins,respectively) into smaller ones, so they can be absorbed by the intestines. Starch molecules, for example, are too large to be absorbed from the intestine, but enzymes hydrolyse the starch chains into smaller molecules such asmaltoseand eventuallyglucose,which can then be absorbed. Different enzymes digest different food substances. Inruminantswhich haveherbivorousdiets, microorganisms in the gut produce another enzyme,cellulaseto break down the cellulose cell walls of plant fiber.[74]

Several enzymes can work together in a specific order, creatingmetabolic pathways.In a metabolic pathway, one enzyme takes the product of another enzyme as a substrate. After the catalytic reaction, the product is then passed on to another enzyme. Sometimes more than one enzyme can catalyze the same reaction in parallel, this can allow more complex regulation: with for example a low constant activity being provided by one enzyme but an inducible high activity from a second enzyme.
Enzymes determine what steps occur in these pathways. Without enzymes, metabolism would neither progress through the same steps, nor be fast enough to serve the needs of the cell. Indeed, a metabolic pathway such asglycolysiscould not exist independently of enzymes. Glucose, for example, can react directly with ATP to becomephosphorylatedat one or more of its carbons. In the absence of enzymes, this occurs so slowly as to be insignificant. However, ifhexokinaseis added, these slow reactions continue to take place except that phosphorylation at carbon 6 occurs so rapidly that if the mixture is tested a short time later,glucose-6-phosphateis found to be the only significant product. Consequently, the network of metabolic pathways within each cell depends on the set of functional enzymes that are present.
Control of activity
There are five main ways that enzyme activity is controlled in the cell.
- Enzyme production(transcriptionandtranslationof enzymegenes) can be enhanced or diminished by a cell in response to changes in the cell's environment. This form ofgene regulationis called enzyme induction and inhibition (seeenzyme induction). For example, bacteria may becomeresistant to antibioticssuch aspenicillinbecause enzymes calledbeta-lactamasesare induced that hydrolyse the crucialbeta-lactam ringwithin the penicillin molecule. Another example are enzymes in thelivercalledcytochrome P450 oxidases,which are important indrug metabolism.Induction or inhibition of these enzymes can causedrug interactions.
- Enzymes can becompartmentalized,with different metabolic pathways occurring in differentcellular compartments.For example,fatty acidsare synthesized by one set of enzymes in thecytosol,endoplasmic reticulumand theGolgi apparatusand used by a different set of enzymes as a source of energy in themitochondrion,throughβ-oxidation.[75]
- Enzymes can be regulated byinhibitorsand activators.For example, the end product(s) of a metabolic pathway are often inhibitors for one of the first enzymes of the pathway (usually the first irreversible step, calledcommitted step), thus regulating the amount of end product made by the pathways. Such a regulatory mechanism is called anegative feedback mechanism,because the amount of the end product produced is regulated by its own concentration. Negative feedback mechanism can effectively adjust the rate of synthesis of intermediate metabolites according to the demands of the cells. This helps allocate materials and energy economically, and prevents the manufacture of excess end products. The control of enzymatic action helps to maintain astable internal environmentin living organisms.
- Enzymes can be regulated throughpost-translational modification.This can includephosphorylation,myristoylationandglycosylation.For example, in the response toinsulin,thephosphorylationof multiple enzymes, includingglycogen synthase,helps control the synthesis or degradation ofglycogenand allows the cell to respond to changes inblood sugar.[76]Another example of post-translational modification is the cleavage of the polypeptide chain.Chymotrypsin,a digestiveprotease,is produced in inactive form aschymotrypsinogenin thepancreasand transported in this form to thestomachwhere it is activated. This stops the enzyme from digesting the pancreas or other tissues before it enters the gut. This type of inactive precursor to an enzyme is known as azymogen.
- Some enzymes may becomeactivated when localized to a different environment(e.g. from a reducing (cytoplasm) to an oxidizing (periplasm) environment, high pH to low pH etc.). For example,hemagglutininin theinfluenzavirus is activated by a conformational change caused by the acidic conditions, these occur when it is taken up inside its host cell and enters thelysosome.[77]
Involvement in disease
Since the tight control of enzyme activity is essential forhomeostasis,any malfunction (mutation, overproduction, underproduction or deletion) of a single critical enzyme can lead to agenetic disease.The importance of enzymes is shown by the fact that a lethal illness can be caused by the malfunction of just one type of enzyme out of the thousands of types present in our bodies.
One example is the most common type ofphenylketonuria.A mutation of a single amino acid in the enzymephenylalanine hydroxylase,which catalyzes the first step in the degradation ofphenylalanine,results in build-up of phenylalanine and related products. This can lead tomental retardationif the disease is untreated.[78]
Another example is whengermline mutationsin genes coding forDNA repairenzymes cause hereditary cancer syndromes such asxeroderma pigmentosum.Defects in these enzymes cause cancer since the body is less able to repair mutations in the genome. This causes a slow accumulation of mutations and results in the development of many types of cancer in the sufferer.
Naming conventions
An enzyme's name is often derived from its substrate or the chemical reaction it catalyzes, with the word ending in-ase.Examples arelactase,alcohol dehydrogenaseandDNA polymerase.This may result in different enzymes, calledisozymes,with the same function having the same basic name. Isoenzymes have a different amino acid sequence and might be distinguished by their optimalpH,kinetic properties or immunologically. Isoenzyme and isozyme are homologous proteins. Furthermore, the normal physiological reaction an enzyme catalyzes may not be the same as under artificial conditions. This can result in the same enzyme being identified with two different names.E.g.Glucose isomerase,used industrially to convertglucoseinto the sweetenerfructose,is a xylose isomerasein vivo.
TheInternational Union of Biochemistry and Molecular Biologyhave developed anomenclaturefor enzymes, theEC numbers;each enzyme is described by a sequence of four numbers preceded by "EC". The first number broadly classifies the enzyme based on its mechanism.
The top-level classification is
- EC 1Oxidoreductases:catalyzeoxidation/reduction reactions
- EC 2Transferases:transfer afunctional group(e.g.a methyl or phosphate group)
- EC 3Hydrolases:catalyze thehydrolysisof various bonds
- EC 4Lyases:cleave various bonds by means other than hydrolysis and oxidation
- EC 5Isomerases:catalyzeisomerizationchanges within a single molecule
- EC 6Ligases:join two molecules withcovalent bonds.
The complete nomenclature can be browsed athttp:// chem.qmul.ac.uk/iubmb/enzyme/.
According to the naming conventions, enzymes are generally classified into six main family classes and many sub-family classes. Some web-servers, e.g., EzyPred [79] and bioinformatics tools have been developed to predict which main family class [80] and sub-family class [81] [82] an enzyme molecule belongs to according to its sequence information alone via thepseudo amino acid composition.
Industrial applications
Enzymes are used in thechemical industryand other industrial applications when extremely specific catalysts are required. However, enzymes in general are limited in the number of reactions they have evolved to catalyze and also by their lack of stability inorganic solventsand at high temperatures. Consequently,protein engineeringis an active area of research and involves attempts to create new enzymes with novel properties, either through rational design orin vitroevolution.[83][84]These efforts have begun to be successful, and a few enzymes have now been desiged "from scratch" to catalyze reactions that do not occur in nature.[85]
| Application | Enzymes used | Uses |
Food processing |
Amylasesfromfungiand plants. | Production of sugars fromstarch,such as in makinghigh-fructose corn syrup.[86]In baking, catalyze breakdown of starch in theflourto sugar. Yeast fermentation of sugar produces the carbon dioxide that raises the dough. |
| Proteases | Biscuit manufacturers use them to lower the protein level of flour. | |
| Baby foods | Trypsin | To predigest baby foods. |
Brewing industry |
Enzymes from barley are released during the mashing stage of beer production. | They degrade starch and proteins to produce simple sugar, amino acids and peptides that are used by yeast for fermentation. |
| Industrially produced barley enzymes | Widely used in the brewing process to substitute for the natural enzymes found in barley. | |
| Amylase, glucanases, proteases | Split polysaccharides and proteins in themalt. | |
| Betaglucanases and arabinoxylanases | Improve the wort and beer filtration characteristics. | |
| Amyloglucosidase and pullulanases | Low-caloriebeerand adjustment of fermentability. | |
| Proteases | Remove cloudiness produced during storage of beers. | |
| Acetolactatedecarboxylase (ALDC) | Increases fermentation efficiency by reducingdiacetylformation.[87] | |
| Fruit juices | Cellulases, pectinases | Clarify fruit juices |
Dairy industry |
Rennin,derived from the stomachs of youngruminant animals(like calves and lambs). | Manufacture of cheese, used tohydrolyzeprotein. |
| Microbially produced enzyme | Now finding increasing use in the dairy industry. | |
| Lipases | Is implemented during the production ofRoquefort cheeseto enhance the ripening of theblue-mould cheese. | |
| Lactases | Break downlactosetoglucoseand galactose. | |
| Meat tenderizers | Papain | To soften meat for cooking. |
| Starch industry | Amylases, amyloglucosideases and glucoamylases | Convertsstarchintoglucoseand varioussyrups. |
| Glucose isomerase | Convertsglucoseintofructosein production ofhigh fructose syrupsfrom starchy materials. These syrups have enhanced sweetening properties and lowercalorific valuesthan sucrose for the same level of sweetness. | |
Paper industry |
Amylases,Xylanases,Cellulasesandligninases | Degrade starch to lowerviscosity,aidingsizingand coating paper. Xylanases reduce bleach required for decolorising; cellulases smooth fibers, enhance water drainage, and promote ink removal; lipases reduce pitch and lignin-degrading enzymes removeligninto soften paper. |
Biofuelindustry |
Cellulases | Used to break down cellulose into sugars that can be fermented (seecellulosic ethanol). |
| Ligninases | Use ofligninwaste | |
| Biological detergent | Primarilyproteases,produced in anextracellularform frombacteria | Used for presoak conditions and direct liquid applications helping with removal of protein stains from clothes. |
| Amylases | Detergentsfor machine dish washing to remove resistant starch residues. | |
| Lipases | Used to assist in the removal of fatty and oily stains. | |
| Cellulases | Used in biologicalfabric conditioners. | |
| Contact lens cleaners | Proteases | To removeproteinsoncontact lensto prevent infections. |
| Rubber industry | Catalase | To generateoxygenfromperoxideto convertlatexinto foam rubber. |
| Photographic industry | Protease(ficin) | Dissolvegelatinoff scrapfilm,allowing recovery of itssilvercontent. |
Molecular biology |
Restriction enzymes,DNA ligaseandpolymerases | Used to manipulate DNA ingenetic engineering,important inpharmacology,agricultureandmedicine.Essential forrestriction digestionand thepolymerase chain reaction.Molecular biology is also important inforensic science. |
See also
- List of enzymes
- Enzyme product
- Enzyme substrate
- Enzyme catalysis
- Enzyme assay
- Protein dynamics
- The Proteolysis Map
- RNA Biocatalysis
- SUMO enzymes
- KiDatabase
- Proteonomicsandprotein engineering
- Immobilized enzyme
- Kinetic Perfection
- Enzyme engineering
References
- ^Smith AL (Ed); et al. (1997).Oxford dictionary of biochemistry and molecular biology.Oxford [Oxfordshire]: Oxford University Press.ISBN0-19-854768-4.
{{cite book}}:Explicit use of et al. in:|author=(help) - ^Grisham, Charles M.; Reginald H. Garrett (1999).Biochemistry.Philadelphia: Saunders College Pub. pp. 426–7.ISBN0-03-022318-0.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Bairoch A. (2000)."The ENZYME database in 2000"(PDF).Nucleic Acids Res.28(1): 304–5.doi:10.1093/nar/28.1.304.PMC102465.PMID10592255.
- ^Lilley D (2005). "Structure, folding and mechanisms of ribozymes".Curr Opin Struct Biol.15(3): 313–23.doi:10.1016/j.sbi.2005.05.002.PMID15919196.
- ^Cech T (2000). "Structural biology. The ribosome is a ribozyme".Science.289(5481): 878–9.doi:10.1126/science.289.5481.878.PMID10960319.
- ^Groves JT (1997). "Artificial enzymes. The importance of being selective".Nature.389(6649): 329–30.doi:10.1038/38602.PMID9311771.
- ^de Réaumur, RAF(1752). "Observations sur la digestion des oiseaux".Histoire de l'academie royale des sciences.1752:266, 461.
- ^Williams, H. S. (1904)A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological SciencesHarper and Brothers (New York) Accessed 4 April 2007
- ^Dubos J. (1951). "Louis Pasteur: Free Lance of Science, Gollancz. Quoted in Manchester K. L. (1995) Louis Pasteur (1822–1895)—chance and the prepared mind".Trends Biotechnol.13(12): 511–5.doi:10.1016/S0167-7799(00)89014-9.PMID8595136.
- ^Kühne coined the word "enzyme" in: W. Kühne (1877) "Über das Verhalten verschiedener organisirter und sog. ungeformter Fermente"(On the behavior of various organized and so-called unformed ferments),Verhandlungen des naturhistorisch-medicinischen Vereins zu Heidelberg,new series, vol. 1, no. 3, pages 190–193. The relevant passage occurs on page 190: "Um Missverständnissen vorzubeugen und lästige Umschreibungen zu vermeiden schlägt Vortragender vor, die ungeformten oder nicht organisirten Fermente, deren Wirkung ohne Anwesenheit von Organismen und ausserhalb derselben erfolgen kann, alsEnzymezu bezeichnen. "(Translation: In order to obviate misunderstandings and avoid cumbersome periphrases, [the author, a university lecturer] suggests designating as" enzymes "the unformed or not organized ferments, whose action can occur without the presence of organisms and outside of the same.)
- ^Nobel Laureate Biography of Eduard Buchner at http://nobelprize.org.Retrieved 4 April 2007.
- ^Text of Eduard Buchner's 1907 Nobel lecture at http://nobelprize.org.Retrieved 4 April 2007.
- ^The naming of enzymes by adding the suffix "-ase" to the substrate on which the enzyme acts, has been traced to French scientistÉmile Duclaux(1840–1904), who intended to honor the discoverers ofdiastase– the first enzyme to be isolated – by introducing this practice in his bookTraité de Microbiologie,vol. 2 (Paris, France: Masson and Co., 1899). See Chapter 1, especially page 9.
- ^1946 Nobel prize for Chemistry laureates at http://nobelprize.org.Retrieved 4 April 2007.
- ^Blake CC, Koenig DF, Mair GA, North AC, Phillips DC, Sarma VR. (1965). "Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution".Nature.22(206): 757–61.doi:10.1038/206757a0.PMID5891407.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Chen LH, Kenyon GL, Curtin F, Harayama S, Bembenek ME, Hajipour G, Whitman CP (1992). "4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer".J. Biol. Chem.267(25): 17716–21.PMID1339435.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Smith S (1 December 1994)."The animal fatty acid synthase: one gene, one polypeptide, seven enzymes".FASEB J.8(15): 1248–59.PMID8001737.
- ^Anfinsen C.B. (1973). "Principles that Govern the Folding of Protein Chains".Science.181(96): 223–30.doi:10.1126/science.181.4096.223.PMID4124164.
- ^Dunaway-Mariano D (2008). "Enzyme function discovery".Structure.16(11): 1599–600.doi:10.1016/j.str.2008.10.001.PMID19000810.
- ^The Catalytic Site Atlas at The European Bioinformatics Institute.Retrieved 4 April 2007.
- ^Jaeger KE, Eggert T. (2004). "Enantioselective biocatalysis optimized by directed evolution".Curr Opin Biotechnol.15(4): 305–13.doi:10.1016/j.copbio.2004.06.007.PMID15358000.
- ^Shevelev IV, Hubscher U. (2002). "The 3' 5' exonucleases".Nat Rev Mol Cell Biol.3(5): 364–76.doi:10.1038/nrm804.PMID11988770.
- ^Tymoczko, John L.; Stryer Berg Tymoczko; Stryer, Lubert; Berg, Jeremy Mark (2002).Biochemistry.San Francisco: W.H. Freeman.ISBN0-7167-4955-6.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Zenkin N, Yuzenkova Y, Severinov K. (2006). "Transcript-assisted transcriptional proofreading".Science.313(5786): 518–20.doi:10.1126/science.1127422.PMID16873663.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Ibba M, Soll D. (2000). "Aminoacyl-tRNA synthesis".Annu Rev Biochem.69:617–50.doi:10.1146/annurev.biochem.69.1.617.PMID10966471.
- ^Rodnina MV, Wintermeyer W. (2001). "Fidelity of aminoacyl-tRNA selection on the ribosome: kinetic and structural mechanisms".Annu Rev Biochem.70:415–35.doi:10.1146/annurev.biochem.70.1.415.PMID11395413.
- ^Firn, Richard."The Screening Hypothesis – a new explanation of secondary product diversity and function".Archived fromthe originalon 2006-05-16.Retrieved2006-10-11.
- ^Fischer E. (1894)."Einfluss der Configuration auf die Wirkung der Enzyme".Ber. Dt. Chem. Ges.27:2985–93.doi:10.1002/cber.18940270364.
{{cite journal}}:line feed character in|journal=at position 9 (help) - ^Koshland D. E. (1958)."Application of a Theory of Enzyme Specificity to Protein Synthesis".Proc. Natl. Acad. Sci.44(2): 98–104.doi:10.1073/pnas.44.2.98.PMC335371.PMID16590179.
- ^Vasella A, Davies GJ, Bohm M. (2002). "Glycosidase mechanisms".Curr Opin Chem Biol.6(5): 619–29.doi:10.1016/S1367-5931(02)00380-0.PMID12413546.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Boyer, Rodney (2002) [2002]. "6".Concepts in Biochemistry(2nd ed.). New York, Chichester, Weinheim, Brisbane, Singapore, Toronto.: John Wiley & Sons, Inc. pp. 137–8.ISBN0-470-00379-0.OCLC51720783.
{{cite book}}:|access-date=requires|url=(help) - ^Savir Y & Tlusty T (2007)."Conformational proofreading: the impact of conformational changes on the specificity of molecular recognition"(PDF).PLoS ONE.2(5): e468.doi:10.1371/journal.pone.0000468.PMC1868595.PMID17520027.
{{cite journal}}:CS1 maint: unflagged free DOI (link) - ^Fersht, Alan (1985).Enzyme structure and mechanism.San Francisco: W.H. Freeman. pp. 50–2.ISBN0-7167-1615-1.
- ^Jencks, William P. (1987).Catalysis in chemistry and enzymology.Mineola, N.Y: Dover.ISBN0-486-65460-5.
- ^Villa J, Strajbl M, Glennon TM, Sham YY, Chu ZT, Warshel A (2000)."How important are entropic contributions to enzyme catalysis?".Proc. Natl. Acad. Sci. U.S.A.97(22): 11899–904.doi:10.1073/pnas.97.22.11899.PMC17266.PMID11050223.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Warshel A, Sharma PK, Kato M, Xiang Y, Liu H, Olsson MH (2006). "Electrostatic basis for enzyme catalysis".Chem. Rev.106(8): 3210–35.doi:10.1021/cr0503106.PMID16895325.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Eisenmesser EZ, Bosco DA, Akke M, Kern D (2002). "Enzyme dynamics during catalysis".Science.295(5559): 1520–3.doi:10.1126/science.1066176.PMID11859194.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Agarwal PK (2005). "Role of protein dynamics in reaction rate enhancement by enzymes".J. Am. Chem. Soc.127(43): 15248–56.doi:10.1021/ja055251s.PMID16248667.
- ^Eisenmesser EZ, Millet O, Labeikovsky W (2005). "Intrinsic dynamics of an enzyme underlies catalysis".Nature.438(7064): 117–21.doi:10.1038/nature04105.PMID16267559.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Yang LW, Bahar I (5 June 2005)."Coupling between catalytic site and collective dynamics: A requirement for mechanochemical activity of enzymes".Structure.13(6): 893–904.doi:10.1016/j.str.2005.03.015.PMC1489920.PMID15939021.
- ^Agarwal PK, Billeter SR, Rajagopalan PT, Benkovic SJ, Hammes-Schiffer S. (5 March 2002)."Network of coupled promoting motions in enzyme catalysis".Proc Natl Acad Sci USA.99(5): 2794–9.doi:10.1073/pnas.052005999.PMC122427.PMID11867722.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Agarwal PK, Geist A, Gorin A (2004). "Protein dynamics and enzymatic catalysis: investigating the peptidyl-prolyl cis-trans isomerization activity of cyclophilin A".Biochemistry.43(33): 10605–18.doi:10.1021/bi0495228.PMID15311922.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Tousignant A, Pelletier JN. (2004). "Protein motions promote catalysis".Chem Biol.11(8): 1037–42.doi:10.1016/j.chembiol.2004.06.007.PMID15324804.
- ^Olsson, MH; Parson, WW; Warshel, A (2006). "Dynamical Contributions to Enzyme Catalysis: Critical Tests of A Popular Hypothesis".Chem. Rev.106(5): 1737–56.doi:10.1021/cr040427e.PMID16683752.
{{cite journal}}:More than one of|author=and|last1=specified (help) - ^Neet KE (1995). "Cooperativity in enzyme function: equilibrium and kinetic aspects".Meth. Enzymol.249:519–67.doi:10.1016/0076-6879(95)49048-5.PMID7791626.
- ^Changeux JP, Edelstein SJ (2005). "Allosteric mechanisms of signal transduction".Science.308(5727): 1424–8.doi:10.1126/science.1108595.PMID15933191.
- ^de Bolster, M.W.G. (1997)."Glossary of Terms Used in Bioinorganic Chemistry: Cofactor".International Union of Pure and Applied Chemistry.Retrieved2007-10-30.
- ^de Bolster, M.W.G. (1997)."Glossary of Terms Used in Bioinorganic Chemistry: Coenzyme".International Union of Pure and Applied Chemistry.Retrieved2007-10-30.
- ^Fisher Z, Hernandez Prada JA, Tu C, Duda D, Yoshioka C, An H, Govindasamy L, Silverman DN and McKenna R. (2005). "Structural and kinetic characterization of active-site histidine as a proton shuttle in catalysis by human carbonic anhydrase II".Biochemistry.44(4): 1097–115.doi:10.1021/bi0480279.PMID15667203.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Wagner, Arthur L. (1975).Vitamins and Coenzymes.Krieger Pub Co.ISBN0-88275-258-8.
- ^BRENDA The Comprehensive Enzyme Information System.Retrieved 4 April 2007.
- ^Törnroth-Horsefield S, Neutze R (2008)."Opening and closing the metabolite gate".Proc. Natl. Acad. Sci. U.S.A.105(50): 19565–6.doi:10.1073/pnas.0810654106.PMC2604989.PMID19073922.
- ^Ferguson, S. J.; Nicholls, David; Ferguson, Stuart (2002).Bioenergetics 3(3rd ed.). San Diego: Academic.ISBN0-12-518121-3.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Henri, V. (1902). "Theorie generale de l'action de quelques diastases".Compt. Rend. Hebd. Acad. Sci. Paris.135:916–9.
- ^Sørensen,P.L. (1909). "Enzymstudien {II}. Über die Messung und Bedeutung der Wasserstoffionenkonzentration bei enzymatischen Prozessen".Biochem. Z.21:131–304.
- ^Michaelis L., Menten M. (1913). "Die Kinetik der Invertinwirkung".Biochem. Z.49:333–369.English translation.Retrieved 6 April 2007.
- ^Briggs G. E., Haldane J. B. S. (1925)."A note on the kinetics of enzyme action".Biochem. J.19(2): 339–339.PMC1259181.PMID16743508.
- ^Radzicka A, Wolfenden R. (1995). "A proficient enzyme".Science.6(267): 90–931.doi:10.1126/science.7809611.PMID7809611.
- ^Ellis RJ (2001). "Macromolecular crowding: obvious but underappreciated".Trends Biochem. Sci.26(10): 597–604.doi:10.1016/S0968-0004(01)01938-7.PMID11590012.
- ^Kopelman R (1988). "Fractal Reaction Kinetics".Science.241(4873): 1620–26.doi:10.1126/science.241.4873.1620.PMID17820893.
- ^Savageau MA (1995). "Michaelis-Menten mechanism reconsidered: implications of fractal kinetics".J. Theor. Biol.176(1): 115–24.doi:10.1006/jtbi.1995.0181.PMID7475096.
- ^Schnell S, Turner TE (2004). "Reaction kinetics in intracellular environments with macromolecular crowding: simulations and rate laws".Prog. Biophys. Mol. Biol.85(2–3): 235–60.doi:10.1016/j.pbiomolbio.2004.01.012.PMID15142746.
- ^Xu F, Ding H (2007). "A new kinetic model for heterogeneous (or spatially confined) enzymatic catalysis: Contributions from the fractal and jamming (overcrowding) effects".Appl. Catal. A: Gen.317(1): 70–81.doi:10.1016/j.apcata.2006.10.014.
- ^Garcia-Viloca M., Gao J., Karplus M., Truhlar D. G. (2004). "How enzymes work: analysis by modern rate theory and computer simulations".Science.303(5655): 186–95.doi:10.1126/science.1088172.PMID14716003.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^
Olsson M. H., Siegbahn P. E., Warshel A. (2004). "Simulations of the large kinetic isotope effect and the temperature dependence of the hydrogen atom transfer in lipoxygenase".J. Am. Chem. Soc.126(9): 2820–8.doi:10.1021/ja037233l.PMID14995199.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Masgrau L., Roujeinikova A., Johannissen L. O., Hothi P., Basran J., Ranaghan K. E., Mulholland A. J., Sutcliffe M. J., Scrutton N. S., Leys D. (2006). "Atomic Description of an Enzyme Reaction Dominated by Proton Tunneling".Science.312(5771): 237–41.doi:10.1126/science.1126002.PMID16614214.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Cleland, W.W. (1963). "The Kinetics of Enzyme-catalyzed Reactions with two or more Substrates or Products 2. {I}nhibition: Nomenclature and Theory".Biochim. Biophys. Acta.67:173–87.
- ^Price, NC. (1979). "What is meant by 'competitive inhibition'?".Trends in Biochemical Sciences.4:pN272.
- ^R Poulin; Lu, L; Ackermann, B; Bey, P; Pegg, AE (1992-01-05)."Mechanism of the irreversible inactivation of mouse ornithine decarboxylase by Alpha -difluoromethylornithine. Characterization of sequences at the inhibitor and coenzyme binding sites".Journal of Biological Chemistry.267(1): 150.PMID1730582.
- ^Yoshikawa S and Caughey WS. (15 May 1990)."Infrared evidence of cyanide binding to iron and copper sites in bovine heart cytochrome c oxidase. Implications regarding oxygen reduction".J Biol Chem.265(14): 7945–58.PMID2159465.
- ^Hunter T. (1995). "Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling".Cell.80(2): 225–36.doi:10.1016/0092-8674(95)90405-0.PMID7834742.
- ^Berg JS, Powell BC, Cheney RE (1 April 2001)."A millennial myosin census".Mol. Biol. Cell.12(4): 780–94.PMC32266.PMID11294886.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Meighen EA (1 March 1991)."Molecular biology of bacterial bioluminescence".Microbiol. Rev.55(1): 123–42.PMC372803.PMID2030669.
- ^Mackie RI, White BA (1 October 1990)."Recent advances in rumen microbial ecology and metabolism: potential impact on nutrient output".J. Dairy Sci.73(10): 2971–95.doi:10.3168/jds.S0022-0302(90)78986-2.PMID2178174.
- ^Faergeman NJ, Knudsen J (1997)."Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling".Biochem. J.323(Pt 1): 1–12.PMC1218279.PMID9173866.
- ^Doble B. W., Woodgett J. R. (2003)."GSK-3: tricks of the trade for a multi-tasking kinase".J. Cell. Sci.116(Pt 7): 1175–86.doi:10.1242/jcs.00384.PMID12615961.
- ^Carr C. M., Kim P. S. (2003). "A spring-loaded mechanism for the conformational change of influenza hemagglutinin".Cell.73(4): 823–32.doi:10.1016/0092-8674(93)90260-W.PMID8500173.
- ^Phenylketonuria: NCBI Genes and Disease.Retrieved 4 April 2007.
- ^Shen H. B., Chou K. C. (2007) EzyPred: A top-down approach for predicting enzyme functional classes and subclasses. Biochem Biophys Res Comm 364, 53–59.
- ^Qiu J. D., Huang J. H., Shi S. P., Liang R. P. (2010) Using the Concept of Chou's Pseudo Amino Acid Composition to Predict Enzyme Family Classes: An Approach with Support Vector Machine Based on Discrete Wavelet Transform. ‘’Protein & Peptide Letters’’ 17, 715–712.
- ^Zhou, X. B., Chen, C., Li, Z. C. & Zou, X. Y. (2007). "Using Chou's amphiphilic pseudo-amino acid composition and support vector machine for prediction of enzyme subfamily classes".Journal of Theoretical Biology.248(3): 546–551.doi:10.1016/j.jtbi.2007.06.001.PMID17628605.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Chou, K. C. (2005). "Using amphiphilic pseudo amino acid composition to predict enzyme subfamily classes".Bioinformatics.21(1): 10–19.doi:10.1093/bioinformatics/bth466.PMID15308540.
- ^Renugopalakrishnan V, Garduno-Juarez R, Narasimhan G, Verma CS, Wei X, Li P. (2005). "Rational design of thermally stable proteins: relevance to bionanotechnology".J Nanosci Nanotechnol.5(11): 1759–1767.doi:10.1166/jnn.2005.441.PMID16433409.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Hult K, Berglund P. (2003). "Engineered enzymes for improved organic synthesis".Curr Opin Biotechnol.14(4): 395–400.doi:10.1016/S0958-1669(03)00095-8.PMID12943848.
- ^Jiang L, Althoff EA, Clemente FR (2008). "De novo computational design of retro-aldol enzymes".Science (journal).319(5868): 1387–91.doi:10.1126/science.1152692.PMID18323453.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Guzmán-Maldonado H, Paredes-López O (1995). "Amylolytic enzymes and products derived from starch: a review".Critical reviews in food science and nutrition.35(5): 373–403.doi:10.1080/10408399509527706.PMID8573280.
- ^Dulieu C, Moll M, Boudrant J, Poncelet D (2000). "Improved performances and control of beer fermentation using encapsulated Alpha -acetolactate decarboxylase and modeling".Biotechnology progress.16(6): 958–65.doi:10.1021/bp000128k.PMID11101321.
{{cite journal}}:CS1 maint: multiple names: authors list (link)
Further reading
|
Etymology and history
Enzyme structure and mechanism
Thermodynamics
|
Kinetics and inhibition
Function and control of enzymes in the cell
Enzyme-naming conventions
Industrial applications
|
External links
- Structure/Function of Enzymes,Web tutorial on enzyme structure and function.
- Enzymes in diagnosisRole of enzymes in diagnosis of diseases.
- Enzyme spotlightMonthly feature at the European Bioinformatics Institute on a selected enzyme.
- AMFEP,Association of Manufacturers and Formulators of Enzyme Products
- BRENDAdatabase, a comprehensive compilation of information and literature references about all known enzymes; requires payment by commercial users.
- Enzyme Structures Databaselinks to the known 3-D structure data of enzymes in theProtein Data Bank.
- ExPASy enzymedatabase, links toSwiss-Protsequence data, entries in other databases and to related literature searches.
- KEGG: Kyoto Encyclopedia of Genes and GenomesGraphical and hypertext-based information on biochemical pathways and enzymes.
- MACiEdatabase of enzyme reaction mechanisms.
- MetaCycdatabase of enzymes and metabolic pathways
- Face-to-Face Interview with Sir John Cornforth who was awarded a Nobel Prize for work on stereochemistry of enzyme-catalyzed reactionsFreeview video by the Vega Science Trust
- Sigma Aldrich Enzyme Assays by Enzyme Name—Hundreds of assays sorted by enzyme name.
- Bugg TD (2001). "The development of mechanistic enzymology in the 20th century".Nat Prod Rep.18(5): 465–93.doi:10.1039/b009205n.PMID11699881.
Template:Link GA Template:Link FA Template:Link FA Template:Link FA Template:Link FA Template:Link FA Template:Link FA Template:Link FA Template:Link FA Template:Link FA


