Methionine
 Skeletal formulaof the canonical form of methionine
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methionine
| |||
| Other names
2-amino-4-(methylthio)butanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | Met, M | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.393 | ||
| EC Number |
| ||
| KEGG |
| ||
PubChemCID
|
|||
| UNII |
| ||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties[2] | |||
| C5H11NO2S | |||
| Molar mass | 149.21g·mol−1 | ||
| Appearance | White crystalline powder | ||
| Density | 1.340 g/cm3 | ||
| Melting point | 281 °C (538 °F; 554 K) decomposes | ||
| Soluble | |||
| Acidity(pKa) | 2.28 (carboxyl), 9.21 (amino)[1] | ||
| Pharmacology | |||
| V03AB26(WHO)QA05BA90(WHO),QG04BA90(WHO) | |||
| Supplementary data page | |||
| Methionine (data page) | |||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Methionine(symbolMetorM)[3](/mɪˈθaɪəniːn/)[4]is anessential amino acidin humans.
As the precursor of other non-essential amino acids such ascysteineandtaurine,versatile compounds such asSAM-e,and the important antioxidantglutathione,methionine plays a critical role in the metabolism and health of many species, including humans. Methionine is also involved inangiogenesisand various processes related to DNA transcription, epigenetic expression, and gene regulation.
Methionine was first isolated in 1921 byJohn Howard Mueller.[5]It isencodedby thecodonAUG. It was named by Satoru Odake in 1925, as an abbreviation of its structural description 2-amino-4-(methylthio)butanoic acid.[6]
Biochemical details
[edit]Methionine (abbreviated asMetorM;encoded by the codon AUG) is an α-amino acidthat is used in thebiosynthesisofproteins.It contains acarboxyl group(which is in the deprotonated −COO−form under biologicalpHconditions), anamino group(which is in theprotonated−NH+
3form under biological pH conditions) located in α-position with respect to the carboxyl group, and anS-methylthioetherside chain, classifying it as anonpolar,aliphaticamino acid.[citation needed]
In nuclear genes ofeukaryotesand inArchaea,methionine is coded for by thestart codon,meaning it indicates the start of thecoding regionand is the first amino acid produced in a nascentpolypeptideduringmRNAtranslation.[7]
A proteinogenic amino acid
[edit]Cysteineand methionine are the twosulfur-containingproteinogenic amino acids.Excluding the few exceptions where methionine may act as aredox sensor(e.g.,methionine sulfoxide[8]), methionine residues do not have a catalytic role.[9]This is in contrast to cysteine residues, where the thiol group has a catalytic role in many proteins.[9]The thioether within methionine does however have a minor structural role due to the stability effect ofS/π interactionsbetween the side chain sulfur atom and aromatic amino acids in one-third of all known protein structures.[9]This lack of a strong role is reflected in experiments where little effect is seen in proteins where methionine is replaced bynorleucine,a straight hydrocarbon sidechain amino acid which lacks the thioether.[10] It has been conjectured that norleucine was present in early versions of the genetic code, but methionine intruded into the final version of the genetic code due to the fact it is used in the cofactorS-adenosylmethionine(SAM-e).[11]This situation is not unique and may have occurred withornithineandarginine.[12]
Encoding
[edit]Methionine is one of only two amino acids encoded by a singlecodon(AUG) in the standardgenetic code(tryptophan,encoded by UGG, is the other). In reflection to the evolutionary origin of its codon, the other AUN codons encodeisoleucine,which is also a hydrophobic amino acid. In the mitochondrial genome of several organisms, includingmetazoaandyeast,the codon AUA also encodes for methionine. In the standard genetic code AUA codes for isoleucine and the respective tRNA (ileXinEscherichia coli) uses the unusual baselysidine(bacteria) oragmatidine(archaea) to discriminate against AUG.[13][14]
The methionine codon AUG is also the most common start codon. A "Start" codon is message for aribosomethat signals the initiation of proteintranslationfrom mRNA when the AUG codon is in aKozak consensus sequence.As a consequence, methionine is often incorporated into theN-terminal position ofproteinsineukaryotesandarchaeaduring translation, although it can be removed bypost-translational modification.Inbacteria,the derivativeN-formylmethionineis used as the initial amino acid.[citation needed]
Derivatives
[edit]S-Adenosylmethionine
[edit]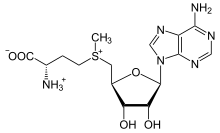
The methionine-derivativeS-adenosylmethionine(SAM-e) is acofactorthat serves mainly as amethyldonor. SAM-e is composed of an adenosyl molecule (via 5′ carbon) attached to the sulfur of methionine, therefore making it asulfoniumcation (i.e., three substituents and positive charge). The sulfur acts as asoft Lewis acid(i.e., donor/electrophile) which allows theS-methyl group to be transferred to an oxygen, nitrogen, or aromatic system, often with the aid of other cofactors such ascobalamin(vitamin B12in humans). Some enzymes use SAM-e to initiate a radical reaction; these are calledradical SAM-eenzymes. As a result of the transfer of the methyl group,S-adenosylhomocysteine is obtained. In bacteria, this is either regenerated by methylation or is salvaged by removing the adenine and the homocysteine, leaving the compound dihydroxypentandione to spontaneously convert intoautoinducer-2,which is excreted as a waste product or quorum signal.[citation needed]
Biosynthesis
[edit]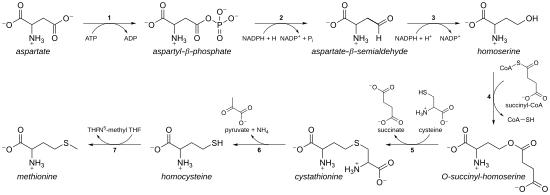
As an essential amino acid, methionine is not synthesizedde novoin humans and other animals, which must ingest methionine or methionine-containing proteins. In plants and microorganisms, methionine biosynthesis belongs to theaspartatefamily, along with threonine andlysine(viadiaminopimelate,but not viaα-aminoadipate). The main backbone is derived fromaspartic acid,while the sulfur may come fromcysteine,methanethiol,orhydrogen sulfide.[9]
- First, aspartic acid is converted via β-aspartyl semialdehyde intohomoserineby two reduction steps of the terminal carboxyl group (homoserine has therefore a γ-hydroxyl, hence thehomo-series). The intermediate aspartate semialdehyde is the branching point with the lysine biosynthetic pathway, where it is insteadcondensedwith pyruvate. Homoserine is the branching point with the threonine pathway, where instead it is isomerised after activating the terminal hydroxyl with phosphate (also used for methionine biosynthesis in plants).[9]
- Homoserine is then activated with a phosphate, succinyl or an acetyl group on the hydroxyl.
- In plants and possibly in some bacteria,[9]phosphate is used. This step is shared with threonine biosynthesis.[9]
- In most organisms, an acetyl group is used to activate the homoserine. This can be catalysed in bacteria by an enzyme encoded bymetXormetA(not homologues).[9]
- Inenterobacteriaand a limited number of other organisms, succinate is used. The enzyme that catalyses the reaction is MetA and the specificity for acetyl-CoA and succinyl-CoA is dictated by a single residue.[9]The physiological basis for the preference of acetyl-CoA or succinyl-CoA is unknown, but such alternative routes are present in some other pathways (e.g.lysine biosynthesis and arginine biosynthesis).
- The hydroxyl activating group is then replaced with cysteine, methanethiol, or hydrogen sulfide. A replacement reaction is technically a γ-eliminationfollowed by a variant of aMichael addition.All the enzymes involved are homologues and members of theCys/Met metabolism PLP-dependent enzyme family,which is a subset of the PLP-dependent fold type I clade. They utilise the cofactor PLP (pyridoxal phosphate), which functions by stabilising carbanion intermediates.[9]
- If it reacts with cysteine, it producescystathionine,which is cleaved to yieldhomocysteine.The enzymes involved arecystathionine-γ-synthase(encoded bymetBin bacteria) andcystathionine-β-lyase(metC). Cystathionine is bound differently in the two enzymes allowing β or γ reactions to occur.[9]
- If it reacts with free hydrogen sulfide, it produces homocysteine. This is catalysed byO-acetylhomoserine aminocarboxypropyltransferase(formerly known asO-acetylhomoserine (thiol)-lyase. It is encoded by eithermetYormetZin bacteria.[9]
- If it reacts with methanethiol, it produces methionine directly. Methanethiol is a byproduct of catabolic pathway of certain compounds, therefore this route is more uncommon.[9]
- If homocysteine is produced, the thiol group is methylated, yielding methionine. Twomethionine synthasesare known; one iscobalamin(vitamin B12) dependent and one is independent.[9]
The pathway using cysteine is called the "transsulfuration pathway",while the pathway using hydrogen sulfide (or methanethiol) is called" direct-sulfurylation pathway ".
Cysteine is similarly produced, namely it can be made from an activated serine and either from homocysteine ( "reverse transsulfurylation route" ) or from hydrogen sulfide ( "direct sulfurylation route" ); the activated serine is generallyO-acetylserine (via CysK or CysM inE. coli), but inAeropyrum pernixand some other archaeaO-phosphoserine is used.[15]CysK and CysM are homologues, but belong to the PLP fold type III clade.[citation needed]
Transsulfurylation pathway
[edit]Enzymes involved in theE. colitranssulfurylation route of methionine biosynthesis:[citation needed]
- Aspartokinase
- Aspartate-semialdehyde dehydrogenase
- Homoserine dehydrogenase
- HomoserineO-transsuccinylase
- Cystathionine-γ-synthase
- Cystathionine-β-lyase
- Methionine synthase(in mammals, this step is performed byhomocysteine methyltransferaseorbetaine—homocysteineS-methyltransferase.)
Other biochemical pathways
[edit]
Although mammals cannot synthesize methionine, they can still use it in a variety of biochemical pathways:
Catabolism
[edit]Methionine is converted toS-adenosylmethionine(SAM-e) by (1)methionine adenosyltransferase.[citation needed]
SAM-e serves as a methyl donor in many (2)methyltransferasereactions, and is converted toS-adenosylhomocysteine(SAH).[citation needed]
(3)Adenosylhomocysteinase cysteine.
Regeneration
[edit]Methionine can be regenerated from homocysteine via (4)methionine synthasein a reaction that requiresvitamin B12as acofactor.[citation needed]
Homocysteine can also be remethylated usingglycine betaine(N,N,N-trimethylglycine, TMG) to methionine via the enzymebetaine-homocysteine methyltransferase(E.C.2.1.1.5, BHMT). BHMT makes up to 1.5% of all the soluble protein of the liver, and recent evidence suggests that it may have a greater influence on methionine and homocysteine homeostasis than methionine synthase.[citation needed]
Reverse-transulfurylation pathway: conversion to cysteine
[edit]Homocysteine can be converted to cysteine.
- (5)Cystathionine-β-synthase(an enzyme which requirespyridoxal phosphate,the active form ofvitamin B6) combines homocysteine and serine to producecystathionine.Instead of degradingcystathionineviacystathionine-β-lyase,as in the biosynthetic pathway, cystathionine is broken down tocysteineandα-ketobutyratevia (6)cystathionine-γ-lyase.[citation needed]
- (7) The enzymeα-ketoacid dehydrogenaseconverts α-ketobutyrate topropionyl-CoA,which is metabolized tosuccinyl-CoAin a three-step process (seepropionyl-CoAfor pathway).[citation needed]
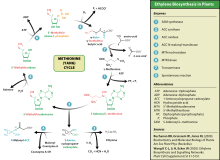
Ethylene synthesis
[edit]Thisamino acidis also used byplantsfor synthesis ofethylene.The process is known as theYangcycle or the methionine cycle.
Metabolic diseases
[edit]The degradation of methionine is impaired in the followingmetabolic diseases:[citation needed]
- Combined malonic and methylmalonic aciduria(CMAMMA)
- Homocystinuria
- Methylmalonic acidemia
- Propionic acidemia
Chemical synthesis
[edit]The industrial synthesis combinesacrolein,methanethiol,and cyanide, which affords thehydantoin.[16]Racemicmethionine can also be synthesized from diethyl sodium phthalimidomalonate by alkylation with chloroethylmethylsulfide (ClCH2CH2SCH3) followed by hydrolysis and decarboxylation. Also see Methanol.[17]
Human nutrition
[edit]There is inconclusive clinical evidence on methionin supplementation.[18]Dietary restriction of methionine can lead to bone-related disorders.[18]
Methionine supplementation may benefit those suffering fromcopper poisoning.[19]
Overconsumption of methionine, themethyl groupdonor inDNA methylation,is related to cancer growth in a number of studies.[20][21]
Requirements
[edit]The Food and Nutrition Board of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) foressential amino acidsin 2002. For methionine combined with cysteine, for adults 19 years and older, 19 mg/kg body weight/day.[22]
This translates to about 1.33 grams per day for a 70 kilogram individual.[citation needed]
Dietary sources
[edit]| Food | g/100 g |
|---|---|
| Egg,white, dried, powder, glucose reduced | 3.204 |
| Sesame seedsflour (low fat) | 1.656 |
| Brazil nuts | 1.124 |
| Cheese,Parmesan, shredded | 1.114 |
| hemp seed,hulled | 0.933 |
| Soy protein concentrate | 0.814 |
| Chicken,broilers or fryers, roasted | 0.801 |
| Fish,tuna, light, canned in water, drained solids | 0.755 |
| Beef,cured, dried | 0.749 |
| Bacon | 0.593 |
| chia seeds | 0.588 |
| Beef,ground, 95% lean meat / 5% fat, raw | 0.565 |
| Pork,ground, 96% lean / 4% fat, raw | 0.564 |
| Soybeans | 0.547 |
| Wheat germ | 0.456 |
| Egg,whole, cooked, hard-boiled | 0.392 |
| Oat | 0.312 |
| Peanuts | 0.309 |
| Chickpea | 0.253 |
| Corn,yellow | 0.197 |
| Almonds | 0.151 |
| Beans, pinto, cooked | 0.117 |
| Lentils,cooked | 0.077 |
| Rice,brown, medium-grain, cooked | 0.052 |
High levels of methionine can be found in eggs, meat, and fish; sesame seeds, Brazil nuts, and some other plant seeds; andcerealgrains. Most fruits and vegetables contain very little. Mostlegumes,though protein dense, are low in methionine. Proteins without adequate methionine are not considered to becomplete proteins.[23]For that reason, racemic methionine is sometimes added as an ingredient topet foods.[24]
Health
[edit]Loss of methionine has been linked to senile greying of hair. Its lack leads to a buildup ofhydrogen peroxideinhair follicles,a reduction intyrosinaseeffectiveness, and a gradual loss of hair color.[25]Methionine raises the intracellular concentration ofglutathione,thereby promoting antioxidant-mediated cell defense and redox regulation. It also protects cells againstdopamineinduced nigral cell loss by binding oxidative metabolites.[26]
Methionine is an intermediate in the biosynthesis ofcysteine,carnitine,taurine,lecithin,phosphatidylcholine,and otherphospholipids.Improper conversion of methionine can lead toatherosclerosis[27]due to accumulation ofhomocysteine.
Other uses
[edit]DL-Methionine is sometimes given as a supplement to dogs; It helps reduce the chances of kidney stones in dogs. Methionine is also known to increase the urinary excretion of quinidine by acidifying the urine. Aminoglycoside antibiotics used to treat urinary tract infections work best in alkaline conditions, and urinary acidification from using methionine can reduce its effectiveness. If a dog is on a diet that acidifies the urine, methionine should not be used.[28]
Methionine is allowed as a supplement to organic poultry feed under the US certified organic program.[29]
Methionine can be used as a nontoxic pesticide option againstgiant swallowtailcaterpillars, which are a serious pest to orange crops.[30]
See also
[edit]- Allantoin
- Formylmethionine
- Methionine oxidation
- Paracetamol poisoning
- Photoreactive methionine
- S-Methylcysteine
References
[edit]- ^Dawson RM, Elliott DC, Elliott WH, Jones KM (1959).Data for Biochemical Research.Oxford: Clarendon Press.
- ^Weast, Robert C., ed. (1981).CRC Handbook of Chemistry and Physics(62nd ed.). Boca Raton, FL: CRC Press. p. C-374.ISBN0-8493-0462-8..
- ^"Nomenclature and Symbolism for Amino Acids and Peptides".IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived fromthe originalon 9 October 2008.Retrieved5 March2018.
- ^"Methionine".Oxford University Press. Archived fromthe originalon January 27, 2018.
- ^Pappenheimer AM (1987)."A Biographical Memoir of John Howard Mueller"(PDF).Washington D.C.: National Academy of Sciences.
- ^Odake, Satoru (1925)."On the Occurrence of a Sulphur-containing Amino acid in Yeast".Bulletin of the Agricultural Chemical Society of Japan.1(8): 87–89.doi:10.1271/bbb1924.1.87.ISSN1881-1272.
- ^Guedes RL, Prosdocimi F, Fernandes GR, Moura LK, Ribeiro HA, Ortega JM (December 2011)."Amino acids biosynthesis and nitrogen assimilation pathways: a great genomic deletion during eukaryotes evolution".BMC Genomics.12(Suppl 4): S2.doi:10.1186/1471-2164-12-S4-S2.PMC3287585.PMID22369087.
- ^Bigelow DJ, Squier TC (January 2005)."Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins".Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics(Submitted manuscript).1703(2): 121–134.doi:10.1016/j.bbapap.2004.09.012.PMID15680220.
- ^abcdefghijklmnFerla MP, Patrick WM (August 2014)."Bacterial methionine biosynthesis".Microbiology.160(Pt 8): 1571–1584.doi:10.1099/mic.0.077826-0.PMID24939187.
- ^Cirino PC, Tang Y, Takahashi K, Tirrell DA, Arnold FH (September 2003). "Global incorporation of norleucine in place of methionine in cytochrome P450 BM-3 heme domain increases peroxygenase activity".Biotechnology and Bioengineering.83(6): 729–734.doi:10.1002/bit.10718.PMID12889037.S2CID11380413.
- ^Alvarez-Carreño C, Becerra A, Lazcano A (October 2013). "Norvaline and norleucine may have been more abundant protein components during early stages of cell evolution".Origins of Life and Evolution of the Biosphere.43(4–5): 363–375.Bibcode:2013OLEB...43..363A.doi:10.1007/s11084-013-9344-3.PMID24013929.S2CID17224537.
- ^Jukes TH (August 1973). "Arginine as an evolutionary intruder into protein synthesis".Biochemical and Biophysical Research Communications.53(3): 709–714.doi:10.1016/0006-291x(73)90151-4.PMID4731949.
- ^Ikeuchi Y, Kimura S, Numata T, Nakamura D, Yokogawa T, Ogata T, Wada T, Suzuki T, Suzuki T (April 2010). "Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea".Nature Chemical Biology.6(4): 277–282.doi:10.1038/nchembio.323.PMID20139989.
- ^Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S (November 1988). "Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification".Nature.336(6195): 179–181.Bibcode:1988Natur.336..179M.doi:10.1038/336179a0.PMID3054566.S2CID4371485.
- ^Mino K, Ishikawa K (September 2003)."A novelO-phospho-L-serine sulfhydrylation reaction catalyzed byO-acetylserine sulfhydrylase fromAeropyrum pernixK1 ".FEBS Letters.551(1–3): 133–138.doi:10.1016/S0014-5793(03)00913-X.PMID12965218.S2CID28360765.
- ^Karlheinz Drauz; Ian Grayson; Axel Kleemann; Hans-Peter Krimmer; Wolfgang Leuchtenberger; Christoph Weckbecker (2006).Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a02_057.pub2.ISBN978-3527306732.
- ^Barger G, Weichselbaum TE (1934)."dl-Methionine ".Organic Syntheses.14:58;Collected Volumes,vol. 2, p. 384.
- ^"Methionine".WebMD.
- ^Cavuoto P, Fenech MF (2012). "A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension".Cancer Treatment Reviews.38(6): 726–736.doi:10.1016/j.ctrv.2012.01.004.PMID22342103.
- ^Cellarier E, Durando X, Vasson MP, Farges MC, Demiden A, Maurizis JC, Madelmont JC, Chollet P (2003). "Methionine dependency and cancer treatment".Cancer Treatment Reviews.29(6): 489–499.doi:10.1016/S0305-7372(03)00118-X.PMID14585259.
- ^Institute of Medicine(2002)."Protein and Amino Acids".Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids.Washington, DC: The National Academies Press. pp. 589–768.doi:10.17226/10490.ISBN978-0-309-08525-0.
- ^Finkelstein JD (May 1990). "Methionine metabolism in mammals".The Journal of Nutritional Biochemistry.1(5): 228–237.doi:10.1016/0955-2863(90)90070-2.PMID15539209.S2CID32264340.
- ^Palika L (1996).The Consumer's Guide to Dog Food: What's in Dog Food, Why It's There and How to Choose the Best Food for Your Dog.New York: Howell Book House.ISBN978-0-87605-467-3.
- ^Wood JM, Decker H, Hartmann H, Chavan B, Rokos H, Spencer JD, et al. (July 2009)."Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair".FASEB Journal.23(7): 2065–75.arXiv:0706.4406.doi:10.1096/fj.08-125435.hdl:10454/6241.PMID19237503.S2CID16069417.
- ^Pinnen F, et al. (2009). "Codrugs linkingL-dopa and sulfur-containing antioxidants: new pharmacological tools against Parkinson's disease ".Journal of Medicinal Chemistry.52(2): 559–63.doi:10.1021/jm801266x.PMID19093882.
- ^Refsum H, Ueland PM, Nygård O, Vollset SE (1998). "Homocysteine and cardiovascular disease".Annual Review of Medicine.49(1): 31–62.doi:10.1146/annurev.med.49.1.31.PMID9509248.
- ^Grimshaw, Jane (July 25, 2011)Methionine for Dogs uses and Side Effects.critters360
- ^"Rules and Regulations".Federal Register.76(49): 13501–13504. March 14, 2011.
- ^Lewis DS, Cuda JP, Stevens BR (December 2011)."A novel biorational pesticide: efficacy of methionine againstHeraclides (Papilio) cresphontes,a surrogate of the invasivePrinceps (Papilio) demoleus(Lepidoptera: Papilionidae) ".Journal of Economic Entomology.104(6): 1986–1990.doi:10.1603/ec11132.PMID22299361.S2CID45255198.
External links
[edit]- Rudra MN, Chowdhury LM (30 September 1950)."Methionine Content of Cereals and Legumes".Nature.166(568): 568.Bibcode:1950Natur.166..568R.doi:10.1038/166568a0.PMID14780151.S2CID3026278.