Atom
The "Scots" that wis uised in this airticle wis written bi a body that haesna a guid grip on the leid. Pleasemak this airticle mair bettergin ye can.(Januar 2021) |
| Helium atom | ||||||||
|---|---|---|---|---|---|---|---|---|
 | ||||||||
| An illustration o theheliumatom, depictin thenucleus(pink) an theelectron clooddistribution (black). The nucleus (upper richt) in helium-4 is in reality spherically symmetric an closely resembles the electron clood, awtho for mair complicatit nuclei this is nae ayeweys the case. The black baur is aneangstrom(10−10mor100pm). | ||||||||
| Clessification | ||||||||
| ||||||||
| Properties | ||||||||
|
Anatomis the smawest consteetuent unit o ordinarymatterthat haes the properties o achemical element.[1]Iverysolit,liquid,gas,anplasmais componed o neutral orionisedatoms. Atoms are very smaw; teepical sizes are aroond 100picometres(a ten-billiont o a metre, in the short scale).[2]
Atomsare smaw eneuchthat attemptin tae predict thair behaviour uisin clessical physics - as if thay war billiard baws, for ensaumple - gies noticeably incorrect predictions due taequantum effects.Throu the development o physics, atomic models hae incorporatitquantum principlestae better expleen an predict the behaviour.
Ivery atom is componed o anucleusan ane or mairelectronsboond tae the nucleus. The nucleus is made o ane or mairprotonsan teepically a seemilar nummer oneutrons.Protons an neutrons are criednucleons.Mair nor 99.94% o an atom's mass is in the nucleus. The protons hae a positiveelectric chairge,the electrons hae a negative electric chairge, an the neutrons hae na electric chairge. If the nummer o protons an electrons are equal, that atom is electrically neutral. If an atom haes mair or fewer electrons nor protons, then it haes an oweraw negative or positive charge, respectively, an it is cried anion.
The electrons o an atom are attractit tae the protons in an atomic nucleus bi thiselectromagnetic force.The protons an neutrons in the nucleus are attractit tae ilk ither bi a different force, thenuclear force,which is uisually stranger than the electromagnetic force repellin the positively charged protons frae ane anither. Unner certaint circumstances the repellin electromagnetic force becomes stranger nor the nuclear force, an nucleons can be ejectit frae the nucleus, leavin behind a different element:nuclear decayresultin innuclear transmutation.
The nummer o protons in the nucleus defines tae whatchemical elementthe atom belangs: for ensaumple, awcopperatoms conteen 29 protons. The nummer o neutrons defines theisotopeo the element.[3]The nummer o electrons influences themagneticproperties o an atom. Atoms can attach tae ane or mair ither atoms bichemical bondstae formchemical compoondssic asmolecules.The abeelity o atoms tae associate an dissociate is responsible for maist o the physical chynges observed in naitur, an is the subject o the discipline ochemistry.
History o atomic theory
[eedit|eedit soorce]Auncient times
[eedit|eedit soorce]The idea that matter is made up o discrete units is a very auld idea, appearin in mony auncient culturs sic as Greece an Indie. The wird "atom" wis creatit biauncient Greek filosofers.Houiver, thir ideas war foondit in filosofical an theological raisonin raither nor evidence an experimentation. As a result, thair views on whit atoms leuk lik an hou thay behave war incorrect.
1800s
[eedit|eedit soorce]
In the early 1800s,John Daltonuised the concept o atoms tae expleen whyelementsayeweys react in ratios o smaw hale nummers (thelaw o multiple proportions). Dalton believed atomic theory coud expleen why watter absorbs different gases in different proportions an aw. For ensaumple, he foond that watter absorbscarbon dioxidefaur better nor it absorbsnitrogen.[4]Dalton hypothesized this wis due tae the differences atween the masses an configurations o the gases' respective pairticles, an carbon dioxide molecules (CO2) are hivier an lairger nor nitrogen molecules (N2). In 1827,botanistRobert Brownuised a microscope tae leuk at dist grains fleetin in watter an discovered that thay muived aboot erratically, a phenomenon that became kent as "Brownian motion".This wis thocht tae be caused bi watter molecules knockin the grains aboot. In 1905,Albert Einsteinpruived the reality o thir molecules an thair motions bi producin the firstStateestical physicsanalysis oBrownian motion.[5][6][7]French physicistJean Perrinuised Einstein's wark tae experimentally determine the mass an dimensions o atoms, thareby conclusively verifeeinDalton's atomic theory.[8]
Early 1900s
[eedit|eedit soorce]The physicistJ. J. Thomsonmeisurt the mass o cathode rays, shawin thay war made o pairticles, but war aroond 1800 times lichter nor the lichtest atom,hydrogen.Tharefore, thay war nae atoms, but a new pairticle, the firstsubatomicpairticle tae be discovered, that he oreeginally cried "corpuscle"but wis later namedelectron,efter pairticles postulatit biGeorge Johnstone Stoneyin 1874. He shawed thay war identical tae pairticles gien off biphotoelectrican radioactive materials an aw.[9]It wis quickly recognised that thay are the pairticles that cairyelectric currentsin metal weirs, an cairy the negative electric chairge within atoms. Thomson wis gien the 1906Nobel Prize in Physicsfor this wark. Sicweys he owerturned the belief that atoms are the indivrrsible, ultimate pairticles o matter.[10]Thomson incorrectly postulatit that the law mass, negatively chairged electrons wra distributed ootthrou the atom in a uniform sea o positive chairge an aw.
In 1909,Hans GeigeranErnest Marsden,unner the direction oErnest Rutherford,bombardit a metal foil wialpha pairticlestae observe hou thay scaitert. Thay expectit aw the alpha pairticles tae pass straicht throu wi little deflection, acause Thomson's model said that the chairges in the atom are sae diffuise that thair electric fields coud nae affect the alpha pairticles muckle. Houiver, Geiger an Marsden spottit alpha pairticles bein deflectit bi angles greater nor 90°, that wis supposed tae be impossible accordin tae Thomson's model. Tae expleen this, Rutherford proponed that the positive charge o the atom is concentratit in a tottie nucleus at the centre o the atom.[11]Rutherford compared his findins tae ane firin a 15-inch shell an it comin back tae hit the person wha fired it.[12]
While experimentin wi the products oradioactive decay,in 1913radiochemistFrederick Soddydiscovered that thare appeared tae be mair nor ane teep o atom at ilk poseetion on the periodic cairt.[13]The termisotopewis coined biMargaret Toddas a suitable name for different atoms that alang tae the same element. J.J. Thomson creatit a technique for separatin atom teeps throu his wark on ionised gases, that subsequently led tae the discovery ostable isotopes.[14]
In 1913 the physicistNiels Bohrproponed a model in that the electrons o an atom war assumed tae orbit the nucleus but coud anerly dae sae in a finite set o orbits, an coud jimp atween thir orbits anerly in discrete cheenges o energy correspondin tae absorption or radiation o a photon.[15]This quantisation wis uised tae expleen why the electrons orbits are stable (gien that normally, chairges in acceleration, includin circular motion, lose kinetic energy that is emittit as electromagnetic radiation, seesynchrotron radiation) an why elements absorb an emit electromagnetic radiation in discrete spectra.[16]Later in the same yearHenry Moseleyprovidit addeetional experimental evidence in favour oNiels Bohr's theory.
In 1924,Louis de Broglieproponed that aw pairticles behave tae an extent lik swaws. In 1926,Erwin Schrödingeruised this idea tae develop a mathematical model o the atom that descrived the electrons as three-dimensionalswawformsraither nor pynt pairticles. The development o themass spectrometerallaed the mass o atoms tae be meisurt wi increased accuracy. The device uises a magnet tae bend the trajectory o a beam o ions, an the amoont o deflection is determined bi the ratio o an atom's mass tae its chairge. The chemistFrancis William Astonuised this instrument tae shaw that isotopes haed different masses. Theatomic masso thir isotopes varied bi integer amoonts, cried thehale nummer rule.[17]The explanation for thir different isotopes awaitit the discovery o theneutron,an unchairged pairticle wi a mass seemilar tae theproton,bi the physicistJames Chadwickin 1932.
In 1938, the German chemistOtto Hahn,a student o Rutherford, directit neutrons ontae uranium atoms expectin tae gettransuranium elements.Insteid, his chemical experiments shawedbariumas a product.[18][19]A year later,Lise Meitneran her neffaeOtto Frischverifee'd that Hahn's result war the first experimentalnuclear fission.[20][21]In 1944, Hahn received theNobel prizein chemistry. Despite Hahn's efforts, the contreibutions o Meitner an Frisch war nae recognised.[22]
Structur
[eedit|eedit soorce]Subatomic pairticles
[eedit|eedit soorce]Tho the wirdatomoreeginally denotit a pairticle that canna be cut intae smawer pairticles, in modren scienteefic uissage the atom is componed o varioussubatomic pairticles.The constituent pairticles o an atom are theelectron,theprotonan theneutron;aw three arefermions.Houiver, thehydrogen-1atom haes na neutrons an thehydron ionhaes na electrons.
The electron is bi faur the least massive o thir pairticles at9.11×10−31kg,wi a negativeelectrical chairgean a size that is too smaw tae be meisurt uisin available techniques.[23]Protons hae a positive chairge an a mass 1,836 times that o the electron, at1.6726×10−27kg.The nummer o protons in an atom is cried itsatomic nummer.Neutrons hae na electrical chairge an hae a free mass o 1,839 times the mass o the electron,[24]or1.6929×10−27kg,the hiviest o the three constituent pairticles, but it can be reduced bi thenuclear bindin energy.
Baith protons an neutrons are composite pairticles componed oelementary pairticlescriedquarks.Thare are twa teeps o quarks in atoms, ilk haein a fractional electric chairge. Protons are componed o twaup quarks(ilk wi chairge +2/3) an anedoun quark(wi a chairge o −1/3). Neutrons conseest o ane up quark an twa doun quarks. This distinction accoonts for the difference in mass an chairge atween the twa pairticles.[25][26]The quarks are held thegither bi thestrang interaction(or strang force), that is mediatit bigluons.
Nucleus
[eedit|eedit soorce]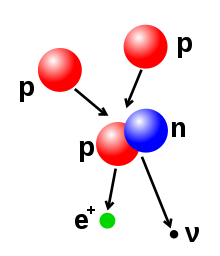
Aw the boond protons an neutrons in an atom mak up a tottieatomic nucleus,an are collectively criednucleons.The radius o a nucleus is approximately equal tae 1.073√Afm,whaurAis the tot nummer o nucleons.[27]This is muckle smawer nor the radius o the atom, that is on the order o 105fm. The nucleons are boond thegither bi a short-ranged attractive potential cried theresidual strang force.At distances smawer nor 2.5 fm this force is muckle mair pouerfu nor theelectrostatic forcethat causes positively chairged protons tae repel ilk ither.[28]The proton, the electron, an the neutron are clessifee'd asfermions.
Electron clood
[eedit|eedit soorce]The electrons in an atom are attractit tae the protons in the nucleus bi theelectromagnetic force.This force binds the electrons inside anelectrostaticpotential wallsurroondin the smawer nucleus, that means that an freemit soorce o energy is needit for the electron tae escape. The closer an electron is tae the nucleus, the greater the attractive force. Hence electrons boond near the centre o the potential wall require mair energy tae escape nor thae at greater separations.
Properties
[eedit|eedit soorce]Bi defineetion, ony twa atoms wi an identical nummer oprotonsin thair nuclei alang tae the samechemical element.Atoms wi equal nummers o protons but a different nummer oneutronsare different isotopes o the same element. For ensaumple, aw hydrogen atoms admit exactly ane proton, but isotopes exist wi no neutrons (hydrogen-1,bi faur the maist common form,[29]cried protium an aw), ane neutron (deuterium), twa neutrons (tritium) anmair nor twa neutrons.
Aboot 339 nuclides occur naiturally onYird,[30]o that 254 (aboot 75%) hae nae been observed tae decay, an are referred tae as "stable isotopes".Houiver, anerly 90 o thir nuclides are stable tae aw decay, evenin theory.Anither 164 (bringin the tot tae 254) hae nae been observed tae decay, even tho in theory it is energetically possible.
The lairge majority o an atom's mass comes frae the protons an neutrons that mak it up. The tot nummer o thir pairticles (cried "nucleons" ) in a gien atom is cried themass nummer.It is a positive integer an dimensionless (insteid o haein dimension o mass), acause it expresses a coont. An ensaumple o uise o a mass nummer is "carbon-12," that haes 12 nucleons (sax protons an sax neutrons). The actualmass o an atom at restis eften expressed uisin theunifee'd atomic mass unit(u), cried dalton (Da) an aw. As even the maist massive atoms are faur too licht tae wirk wi directly, chemists insteid uise the unit omoles.Ane mole o atoms o ony element ayeweys haes the same nummer o atoms (aboot6.022×1023). This nummer wis chosen sae that if an element haes an atomic mass o 1 u, a mole o atoms o that element haes a mass close tae ane gram.
Atoms lack a well-defined ooter boondary, sae thair dimensions are uisually descrived in terms o anatomic radius.This is a meisur o the distance oot tae that the electron clood extends frae the nucleus.[2]Whan subjectit tae fremmit forces, likelectrical fields,the shape o an atom mey deviate fraespherical symmetry.The deformation depends on the field magnitude an the orbital teep o ooter shell electrons, as shawn bigroup-theoreticalconseederations.
Atomic dimensions are thoosands o times smawer nor the swawlenths olicht(400–700nm) sae thay canna be viewed uising anoptical microscope.Houever, individual atoms can be observed uisin ascannin tunnelin microscope.Tae veesualise the size o the atom, consider that a teepical human hair is aboot 1 million carbon atoms in weenth.[31]A single drap o watter conteens aboot 2sextillion(2×1021) atoms o oxygen, an twice the nummer o hydrogen atoms.[32]
Ivery element haes ane or mair isotopes that hae unstable nuclei that are subject tae radioactive decay, causin the nucleus tae emit pairticles or electromagnetic radiation. Radioactivity can occur whan the radius o a nucleus is lairge compared wi the radius o the strang force, that anerly acts ower distances on the order o 1 fm.[33]
The maist common forms o radioactive decay are:[34][35]
- Alpha decay:this process is caused whan the nucleus emits an alpha pairticle, that is a helium nucleus conseestin o twa protons an twa neutrons. The result o the emission is a new element wi a laweratomic nummer.
- Beta decay(anelectron captur): thir processes are regulatit bi thewaik force,an result frae a transformation o a neutron intae a proton, or a proton intae a neutron. The neutron tae proton transeetion is accompanied bi the emission o an electron an anantineutrino,while proton tae neutron transeetion (except in electron captur) causes the emission o apositronan aneutrino.The electron or positron emissions are cried beta pairticles. Beta decay aither increases or decreases the atomic nummer o the nucleus bi ane. Electron captur is mair common nor positron emission, acause it requires less energy. In this teep o decay, an electron is absorbed bi the nucleus, raither nor a positron emittit frae the nucleus. A neutrino is still emittit in this process, an a proton cheenges tae a neutron.
- Gamma decay:this process results frae a cheenge in the energy level o the nucleus tae a lawer state, resultin in the emission o electromagnetic radiation. The excitit state o a nucleus which results in gamma emission uisually occurs follaein the emission o an alpha or a beta pairticle. Sicwweys, gamma decay uisually follaes alpha or beta decay.
Elementar pairticles possess an intrinsic quantum mechanical property kent asspin.This is analogous tae theangular momentumo an object that is spinnin aroond itscentre o mass,awtho strictly speakin thir pairticles are believed tae be pynt-lik an canna be said tae be rotatin. Spin is meisurt in units o the reducedPlanck constant(ħ), wi electrons, protons an neutrons aw haein spin ½ ħ, or "spin-½".
At temperaturs close taeabsolute zero,atoms can form aBose–Einstein condensate,at which pynt quantum mechanical effects, that are normally anerly observed at the atomic scale,becom apparent on a macroscopic scale.[36][37]This super-cuiled collection o atoms then behaves as a singlesuper atom,that mey allae fundamental checks o quantum mechanical behaviour.[38]
Identification
[eedit|eedit soorce]Thescannin tunnelin microscopeis a device for viewin surfaces at the atomic level. It uises thequantum tunnelinphenomenon, that allaes pairticles tae pass throu a barrier that wad normally be insurmoontable. Electrons tunnel throu the vacuum atween twa planar metal electrodes, on ilk o that is anadsorbedatom, providin a tunnelin-current density that can be meisurt. Scannin ane atom (taken as the tip) as it muives past the ither (the saumple) permits plottin o tip displacement versus lateral separation for a constant current. The calculation shaws the extent tae that scannin-tunnelin-microscope eemages o an individual atom are veesible. It confirms that for law bias, the microscope eemages the space-averaged dimensions o the electron orbitals athort closely packed energy levels—theFermi levellocal density o states.[39][40]
References
[eedit|eedit soorce]- ↑"Atom".Compendium of Chemical Terminology (IUPAC Gold Book)(2nd ed.). IUPAC.Retrieved25 Apryle2015.
- ↑abGhosh, D. C.; Biswas, R. (2002)."Theoretical calculation of Absolute Radii of Atoms and Ions. Part 1. The Atomic Radii".Int. J. Mol. Sci.3:87–113.doi:10.3390/i3020087.
- ↑Leigh, G. J., ed. (1990).International Union of Pure and Applied Chemistry, Commission on the Nomenclature of Inorganic Chemistry,Nomenclature of Organic Chemistry– Recommendations 1990.Oxford: Blackwell Scientific Publications. p. 35.ISBN0-08-022369-9.
An atom is the smallest unit quantity of an element that is capable of existence whether alone or in chemical combination with other atoms of the same or other elements.
- ↑Dalton, John. "On the Absorption of Gases by Water and Other Liquids",inMemoirs of the Literary and Philosophical Society of Manchester.1803. Retrieved on August 29, 2007.
- ↑Einstein, Albert (1905)."Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen"(PDF).Annalen der Physik(in German).322(8): 549–560.Bibcode:1905AnP...322..549E.doi:10.1002/andp.19053220806.Archived fraethe original(PDF)on 18 Julie 2007.Retrieved4 Februar2007.
- ↑Mazo, Robert M. (2002).Brownian Motion: Fluctuations, Dynamics, and Applications.Oxford University Press. pp.1–7.ISBN0-19-851567-7.OCLC48753074.
- ↑Lee, Y.K.; Hoon, K. (1995)."Brownian Motion".Imperial College.Archived fraethe originalon 18 December 2007.Retrieved18 December2007.Unknown parameter
|deadurl=ignored (help) - ↑Patterson, G. (2007)."Jean Perrin and the triumph of the atomic doctrine".Endeavour.31(2): 50–53.doi:10.1016/j.endeavour.2007.05.003.PMID17602746.
- ↑Thomson, J. J. (August 1901)."On bodies smaller than atoms".The Popular Science Monthly.Bonnier Corp.: 323–335.Retrieved21 Juin2009.
- ↑"J.J. Thomson".Nobel Foundation.1906.Retrieved20 December2007.
- ↑Rutherford, E. (1911)."The Scattering of α and β Particles by Matter and the Structure of the Atom"(PDF).Philosophical Magazine.21(125): 669–88.doi:10.1080/14786440508637080.
- ↑"The Gold Foil Experiment".myweb.usf.edu.Archived fraethe originalon 19 November 2016.Retrieved2 August2017.
- ↑"Frederick Soddy, The Nobel Prize in Chemistry 1921".Nobel Foundation.Retrieved18 Januar2008.
- ↑Thomson, Joseph John (1913)."Rays of positive electricity".Proceedings of the Royal Society.A.89(607): 1–20.Bibcode:1913RSPSA..89....1T.doi:10.1098/rspa.1913.0057.
- ↑Stern, David P. (16 Mey 2005)."The Atomic Nucleus and Bohr's Early Model of the Atom".NASA/Goddard Space Flight Center.Archived fraethe originalon 20 August 2007.Retrieved20 December2007.
- ↑Bohr, Niels (11 December 1922)."Niels Bohr, The Nobel Prize in Physics 1922, Nobel Lecture".Nobel Foundation.Retrieved16 Februar2008.
- ↑Aston, Francis W. (1920). "The constitution of atmospheric neon".Philosophical Magazine.39(6): 449–55.doi:10.1080/14786440408636058.
- ↑Bowden, Mary Ellen (1997). "Otto Hahn, Lise Meitner, and Fritz Strassmann".Chemical achievers: the human face of the chemical sciences.Philadelphia, PA: Chemical Heritage Foundation. pp.76–80, 125.ISBN9780941901123.
- ↑"Otto Hahn, Lise Meitner, and Fritz Strassmann".Chemical Heritage Foundation.Retrieved27 October2016.
- ↑Meitner, Lise; Frisch, Otto Robert (1939). "Disintegration of uranium by neutrons: a new type of nuclear reaction".Nature.143(3615): 239–240.Bibcode:1939Natur.143..239M.doi:10.1038/143239a0.
- ↑Schroeder, M."Lise Meitner – Zur 125. Wiederkehr Ihres Geburtstages"(in German). Archived fraethe originalon 19 Julie 2011.Retrieved4 Juin2009.
- ↑Crawford, E.; Sime, Ruth Lewin; Walker, Mark (1997)."A Nobel tale of postwar injustice".Physics Today.50(9): 26–32.Bibcode:1997PhT....50i..26C.doi:10.1063/1.881933.
- ↑Demtröder, Wolfgang (2002).Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics(1st ed.). Springer. pp. 39–42.ISBN3-540-20631-0.OCLC181435713.
- ↑Woan, Graham (2000).The Cambridge Handbook of Physics.Cambridge University Press. p.8.ISBN0-521-57507-9.OCLC224032426.
- ↑Particle Data Group (2002)."The Particle Adventure".Lawrence Berkeley Laboratory. Archived fraethe originalon 4 Januar 2007.Retrieved3 Januar2007.Unknown parameter
|deadurl=ignored (help) - ↑Schombert, James (18 Apryle 2006)."Elementary Particles".University of Oregon. Archived fraethe originalon 30 August 2011.Retrieved3 Januar2007.
- ↑Jevremovic, Tatjana (2005).Nuclear Principles in Engineering.Springer. p.63.ISBN0-387-23284-2.OCLC228384008.
- ↑Pfeffer, Jeremy I.; Nir, Shlomo (2000).Modern Physics: An Introductory Text.Imperial College Press. pp. 330–336.ISBN1-86094-250-4.OCLC45900880.
- ↑Matis, Howard S. (9 August 2000)."The Isotopes of Hydrogen".Guide to the Nuclear Wall Chart.Lawrence Berkeley National Lab. Archived fraethe originalon 18 December 2007.Retrieved21 December2007.Unknown parameter
|deadurl=ignored (help) - ↑Lindsay, Don (30 Julie 2000)."Radioactives Missing From The Earth".Don Lindsay Archive. Archived fraethe originalon 28 Apryle 2007.Retrieved23 Mey2007.Unknown parameter
|deadurl=ignored (help) - ↑Staff (2007)."Small Miracles: Harnessing nanotechnology".Oregon State University.Retrieved7 Januar2007.—describes the width of a human hair as105nmand 10 carbon atoms as spanning 1 nm.
- ↑Padilla, Michael J.; Miaoulis, Ioannis; Cyr, Martha (2002).Prentice Hall Science Explorer: Chemical Building Blocks.Upper Saddle River, New Jersey USA: Prentice-Hall, Inc. p. 32.ISBN0-13-054091-9.OCLC47925884.
There are 2,000,000,000,000,000,000,000 (that's 2 sextillion) atoms of oxygen in one drop of water—and twice as many atoms of hydrogen.
- ↑"Radioactivity".Splung.com. Archived fraethe originalon 4 December 2007.Retrieved19 December2007.Unknown parameter
|deadurl=ignored (help) - ↑L'Annunziata, Michael F. (2003).Handbook of Radioactivity Analysis.Academic Press. pp.3–56.ISBN0-12-436603-1.OCLC16212955.
- ↑Firestone, Richard B. (22 Mey 2000)."Radioactive Decay Modes".Berkeley Laboratory. Archived fraethe originalon 29 September 2006.Retrieved7 Januar2007.Unknown parameter
|deadurl=ignored (help)Archived2006-09-29 at theWayback Machine - ↑Myers, Richard (2003).The Basics of Chemistry.Greenwood Press. p.85.ISBN0-313-31664-3.OCLC50164580.
- ↑Staff (9 October 2001)."Bose-Einstein Condensate: A New Form of Matter".National Institute of Standards and Technology. Archived fraethe originalon 3 Januar 2008.Retrieved16 Januar2008.Unknown parameter
|deadurl=ignored (help) - ↑Colton, Imogen; Fyffe, Jeanette (3 Februar 1999)."Super Atoms from Bose-Einstein Condensation".The University of Melbourne. Archived fraethe originalon 29 August 2007.Retrieved6 Februar2008.Archived2007-08-29 at theWayback Machine
- ↑Jacox, Marilyn; Gadzuk, J. William (November 1997)."Scanning Tunneling Microscope".National Institute of Standards and Technology. Archived fraethe originalon 7 Januar 2008.Retrieved11 Januar2008.Unknown parameter
|deadurl=ignored (help) - ↑"The Nobel Prize in Physics 1986".The Nobel Foundation.Retrieved11 Januar2008.—in particular, see the Nobel lecture by G. Binnig and H. Rohrer.
| Wikimedia Commons haes media relatit taeAtoms. |
| This science-relatit airticle is astub.Ye can help Wikipaedia biexpandin it. |
