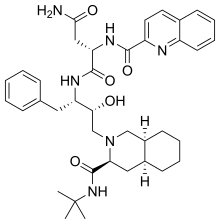
Sakvinavir
Klinički podaci
Robne marke
Fortovase, Invirase, ROC
AHFS/Drugs.com
Monografija
Identifikatori
CAS broj
127779-20-8
ATC kod
J05 AE01
PubChem [1] [2] 60787
DrugBank
DB01232
ChemSpider [3] 54783
ChEMBL [4] CHEMBL114 Y
Hemijski podaci
Formula
C 38 H 50 N 6 O 5
Mol. masa
670,841
SMILES
eMolekuli PubHem
InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26?,27?,30-,31-,32-,33+/m0/s1Y
Fizički podaci
Tačka topljenja
349.84 °C (662 °F)
Farmakokinetički podaci
Izlučivanje
Renalno
Farmakoinformacioni podaci
Trudnoća
?
Pravni status
Način primene
Oralno
Sakvinavir jeorgansko jedinjenje ,koje sadrži 38atoma ugljenika i imamolekulsku masu od 670,841Da .[5] [6] [7]
↑ Li Q, Cheng T, Wang Y, Bryant SH (2010).„PubChem as a public resource for drug discovery.” .Drug Discov Today 15 (23-24): 1052-7.DOI :10.1016/j.drudis.2010.10.003 .PMID 20970519 . edit ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”.Annual Reports in Computational Chemistry 4 :217-241.DOI :10.1016/S1574-1400(08)00012-1 . ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010).„Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining” .J Cheminform 2 (1): 3.DOI :10.1186/1758-2946-2-3 .PMID 20331846 . edit ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”.Nucleic Acids Res 40 (Database issue): D1100-7.DOI :10.1093/nar/gkr777 .PMID 21948594 . edit ↑ Forestier F, de Renty P, Peytavin G, Dohin E, Farinotti R, Mandelbrot L: Maternal-fetal transfer of saquinavir studied in the ex vivo placental perfusion model. Am J Obstet Gynecol. 2001 Jul;185(1):178-81.PMID 11483925
↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011).„DrugBank 3.0: a comprehensive resource for omics research on drugs” .Nucleic Acids Res. 39 (Database issue): D1035-41.DOI :10.1093/nar/gkq1126 .PMC 3013709 .PMID 21059682 . ↑ David S. Wishart, Craig Knox, An Chi Guo, Dean Cheng, Savita Shrivastava, Dan Tzur, Bijaya Gautam, and Murtaza Hassanali (2008).„DrugBank: a knowledgebase for drugs, drug actions and drug targets” .Nucleic Acids Res 36 (Database issue): D901-6.DOI :10.1093/nar/gkm958 .PMC 2238889 .PMID 18048412 . ↑ Ghose, A.K., Viswanadhan V.N., and Wendoloski, J.J. (1998).„Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods” .J. Phys. Chem. A 102 :3762-3772.DOI :10.1021/jp980230o . ↑ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE. (2001).„Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices” .Chem Inf. Comput. Sci. 41 :1488-1493.DOI :10.1021/ci000392t .PMID 11749573 . ↑ Ertl P., Rohde B., Selzer P. (2000).„Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties” .J. Med. Chem. 43 :3714-3717.DOI :10.1021/jm000942e .PMID 11020286 .