Agomelatin
Izgled

| |||
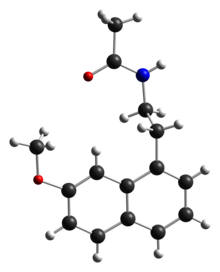
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| N-[2-(7-metoksinaftalen-1-il)etil]acetamid | |||
| Klinički podaci | |||
| AHFS/Drugs | Internacionalno ime leka | ||
| Identifikatori | |||
| CAS broj | 138112-76-2 | ||
| ATC kod | N06AX22 | ||
| PubChem[1][2] | 82148 | ||
| ChemSpider[3] | 74141 | ||
| UNII | 137R1N49AD | ||
| KEGG[4] | D02578 | ||
| ChEMBL[5] | CHEMBL10878 | ||
| Hemijski podaci | |||
| Formula | C15H17NO2 | ||
| Mol. masa | 243,301 | ||
| SMILES | eMolekuli&PubHem | ||
| |||
| Farmakokinetičkipodaci | |||
| Bioraspoloživost | <5% | ||
| Metabolizam | hepatički (90% CYP1A2; 10% CYP2C9) | ||
| Poluvreme eliminacije | < 2 h | ||
| Farmakoinformacioni podaci | |||
| Licenca | |||
| Trudnoća | ? | ||
| Pravni status | ℞Prescription only | ||
| Način primene | Oralno | ||
Agomelatin(Valdoksan,Melitor,Timanaks) jeantidepresiv.On je u prodaji za tretmankliničke depresije.[6][7]Poznato je da ima manje izražene seksualne nuspojave, kao i slabije diskontinuacione efekte u poređenju sa drugim antidepresivima.
Agomelatin je melatonergički agonist (MT1(Ki=0.10nM±0.01nM) iMT2receptori (Ki=0.12nM±0.02nM)) i5-HT2Cantagonist(IC50=270nM; pKi=6.15±0.04). Iz studija vezivanja proizilazi da on nema uticaja na unos monoamina i da nema afiniteta za adrenergičke, histaminergičke, holinergičke, dopaminergičke i benzodiazepinske receptore, niti druge serotonergičke receptore.[8]
- ↑Li Q, Cheng T, Wang Y, Bryant SH (2010).„PubChem as a public resource for drug discovery.”.Drug Discov Today15(23-24): 1052-7.DOI:10.1016/j.drudis.2010.10.003.PMID20970519.
- ↑Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”.Annual Reports in Computational Chemistry4:217-241.DOI:10.1016/S1574-1400(08)00012-1.
- ↑Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010).„Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”.J Cheminform2(1): 3.DOI:10.1186/1758-2946-2-3.PMID20331846.
- ↑Joanne Wixon, Douglas Kell (2000).„Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”.Yeast17(1): 48–55.DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”.Nucleic Acids Res40(Database issue): D1100-7.DOI:10.1093/nar/gkr777.PMID21948594.
- ↑Hardman JG, Limbird LE, Gilman AG. (2001).Goodman & Gilman's The Pharmacological Basis of Therapeutics(10 izd.). New York: McGraw-Hill.DOI:10.1036/0071422803.ISBN0-07-135469-7.
- ↑Pdr Staff (2009).PDR: Physicians Desk Reference 2010 (Physicians' Desk Reference (Pdr)).Rozelle, N.S.W: Thomson Reuters.ISBN1-56363-748-0.
- ↑„Summary of Product Characteristics”(PDF). European Medicine Agency. 2003. Arhivirano izoriginalana datum 2014-10-29.Pristupljeno 22. 9. 2010.
{{refbegin|2}{
- Official product siteArhivirano2019-07-29 naWayback Machine-u
- Manufacturer web site
- Agomelatine Psychonauts Google Group[mrtav link]
- Novartis pipelineArhivirano2011-02-16 naWayback Machine-u
- Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors
- The Novel Melatonin Agonist Agomelatine (S20098) Is an Antagonist at 5-Hydroxytryptamine2C Receptors, Blockade of Which Enhances the Activity of Frontocortical Dopaminergic and Adrenergic Pathways
- Agomelatine treatment has promising results in transgenic murine model
