Proton
Aprotonis part of anatom.[1]They are found in thenucleusof an atom along withneutrons.[1]Theperiodic tablegroups atoms according to how many protons they have. A single atom ofhydrogen(the lightest kind of atom) is made up of anelectronmoving around a proton. Most of themassof this atom is in the proton, which is almost 2000 times heavier than the electron. Protons andneutronsmake up the nucleus of every other kind of atom. In any oneelement,the number of protons is always the same. Scientists discovered in the early 20th century that an atom'satomicnumber is equal to the number of protons in that atom.
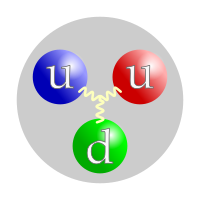
Protons are made ofquarks.[1]A proton is believed to be made up of 3 quarks, twoup quarksand onedown quark.[1]One down quark has achargeof -1/3, and two up quarks have a charge of +2/3 each. This adds to a charge of +1. A proton has a very smallmass.The mass of the proton is about oneatomic mass unit.The mass of the neutron is also about one atomic mass unit. The size of a proton is determined by the vibration of the quarks that are in it, and these quarks effectively form a cloud. This means that a proton is not so much a hard ball as an area that contains quarks.
Related pages
[change|change source]References
[change|change source]- ↑1.01.11.21.3Cox, Brian; Cohen, Andrew (2011).Wonders of the Universe.HarperCollins. p.108-109.ISBN9780007395828.
