Oxygen
 Liquid oxygen boiling | |||||||||||||||||||||||||||||||
| Oxygen | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | O2,O3(ozone) and more (seeAllotropes of oxygen) | ||||||||||||||||||||||||||||||
| Appearance | (O2) gas: colourless liquid and solid: pale blue | ||||||||||||||||||||||||||||||
| Standard atomic weightAr°(O) | |||||||||||||||||||||||||||||||
| [15.99903,15.99977][1] | |||||||||||||||||||||||||||||||
| Abundance | |||||||||||||||||||||||||||||||
| in theEarth's crust | 46% | ||||||||||||||||||||||||||||||
| in theoceans | 86% | ||||||||||||||||||||||||||||||
| in thesolar system | 1% | ||||||||||||||||||||||||||||||
| Oxygen in theperiodic table | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| Group | group 16 (chalcogens) | ||||||||||||||||||||||||||||||
| Period | period 2 | ||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||
| Electron configuration | [He] 2s22p4 | ||||||||||||||||||||||||||||||
| Electrons per shell | 2, 6 | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| PhaseatSTP | gas(O2) | ||||||||||||||||||||||||||||||
| Melting point | 54.36K(−218.79 °C, −361.82 °F) | ||||||||||||||||||||||||||||||
| Boiling point | 90.188 K (−182.962 °C, −297.332 °F) | ||||||||||||||||||||||||||||||
| Density(at STP) | 1.429 g/L | ||||||||||||||||||||||||||||||
| when liquid (atb.p.) | 1.141 g/cm3 | ||||||||||||||||||||||||||||||
| Triple point | 54.361 K, 0.1463 kPa | ||||||||||||||||||||||||||||||
| Critical point | 154.581 K, 5.043 MPa | ||||||||||||||||||||||||||||||
| Heat of fusion | (O2) 0.444kJ/mol | ||||||||||||||||||||||||||||||
| Heat of vaporization | (O2) 6.82 kJ/mol | ||||||||||||||||||||||||||||||
| Molar heat capacity | (O2) 29.378 J/(mol·K) | ||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||
| Oxidation states | −2,−1,0,+1,+2 | ||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 3.44 | ||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||
| Covalent radius | 66±2pm | ||||||||||||||||||||||||||||||
| Van der Waals radius | 152 pm | ||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||
| Crystal structure | cubic | ||||||||||||||||||||||||||||||
| Speed of sound | 330m/s(gas, at 27 °C) | ||||||||||||||||||||||||||||||
| Thermal conductivity | 26.58×10−3W/(m⋅K) | ||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +3449.0·10−6cm3/mol (293 K)[2] | ||||||||||||||||||||||||||||||
| CAS Number | 7782-44-7 | ||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||
| Discovery | Carl Wilhelm Scheele(1771) | ||||||||||||||||||||||||||||||
| Named by | Antoine Lavoisier(1777) | ||||||||||||||||||||||||||||||
| Isotopes of oxygen | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
Oxygenis achemical element.It has the symbolOandatomic number8. It is the third most commonelementin theuniverse,afterhydrogenandhelium.
Oxygen ismore than a fifthof theEarth's atmosphereby volume. In theair,two oxygenatomsusuallyjointo make dioxygen (O
2), acolourlessgas.This gas is often just called oxygen. It has notasteorsmell.It is palebluewhen it isliquidorsolid.
Oxygen is part of thechalcogengroupon theperiodic table.It is a veryreactivenonmetal.It makesoxidesand othercompoundswith many elements. The oxygen in these oxides and in other compounds (mostlysilicateminerals,andcalcium carbonateinlimestone) makes upnearly halfof theEarth's crust,bymass.
Most living things use oxygen inrespiration.Manymoleculesin living things have oxygen in them, such asproteins,nucleic acids,carbohydratesandfats.Oxygen is a part ofwater,which all known life needs to live.Algae,cyanobacteriaandplantsmake the Earth's oxygen gas byphotosynthesis.They use theSun's light to get hydrogen from water, giving off oxygen.
At the top of the Earth's atmosphere isozone(O
3), in theozone layer.It absorbsultraviolet radiation,which means less radiation reaches ground level.
Oxygen gas is used for makingsteel,plasticsandtextiles.It also has medical uses and is used for breathing when there is no good air (bydiversandfirefighters,for example), and forwelding.Liquid oxygen and oxygen-rich compounds can be used as arocket propellant.
History[change|change source]
Oxygen gas (O
2) wasisolatedbyMichael Sendivogiusbefore 1604. It is often thought that the gas was discovered in 1773 byCarl Wilhelm Scheele,inSweden,or in 1774 byJoseph Priestley,inEngland.Priestley is usually thought to be the main discoverer because his work was published first (although he called it "dephlogisticated air",and did not think it was achemical element).Antoine Lavoisiergave the nameoxygèneto the gas in 1777. He was the first person to say it was a chemical element. He was also right about how it helpscombustionwork.
Early experiments[change|change source]
One of the first knownexperimentson howcombustionneedsairwas carried out byGreekPhilo of Byzantiumin the 2nd centuryBC.He wrote in his workPneumaticathat turning avesselupside down over a burningcandleand putting water around this vessel meant that some water went into the vessel.[3]Philo thought this was because the air was turned into theclassical elementfire. This is wrong. A long time after,Leonardo da Vinciworked out that some air was used up during combustion, and this forced water into the vessel.[4]
In the late 17th century,Robert Boylefound that air is needed for combustion.EnglishchemistJohn Mayowadded to this by showing that fire only needed a part of air. We now call this oxygen (O2).[5]He found that a candle burning in a closed container made the water rise to replace a fourteenth of the air'svolumebefore it went out.[6]The same thing happened when a livemousewas put into the box. From this, he worked out that oxygen is used for both respiration and combustion.
Phlogiston theory[change|change source]
Robert Hooke,Ole Borch,Mikhail LomonosovandPierre Bayenall made oxygen in experiments in the 17th and 18th centuries. None of them thought it was achemical element.[7]This was probably because of the idea of thephlogiston theory.This was what most people believed caused combustion andcorrosion.[8]
J. J. Bechercame up with the theory in 1667, andGeorg Ernst Stahladded to it in 1731.[9]Thephlogiston theorystated that all combustible materials were made of two parts. One part, called phlogiston, was given off when the substance containing it was burned.[4]
Materials that leave very littleresiduewhen they burn, likewoodorcoal,were thought to be made mostly of phlogiston. Things thatcorrode,likeiron,were thought to contain very little. Air was not part of this theory.[4]
Discovery[change|change source]
Polishalchemist,philosopherandphysicianMichael Sendivogiuswrote about something in air that he called the "food of life",[10]and this meant what we now call oxygen.[11]Sendivogius found, between 1598 and 1604, that the substance in air is the same as he got by heatingpotassium nitrate.Some people believe this was the discovery of oxygen while others disagree. Some say that oxygen was discovered bySwedishpharmacistCarl Wilhelm Scheele.He got oxygen in 1771 by heatingmercuric oxideand somenitrates.[4][12][13]Scheele called the gas "fire air", because it was the only gas known to allow combustion (gases were called "airs" at this time). He published his discovery in 1777.[14]
On 1 August 1774,BritishclergymanJoseph Priestleyfocused sunlight onmercuric oxidein aglasstube. From thisexperimenthe got a gas that he called "dephlogisticated air".[13]He found that candles burned more brightly in the gas and a mouse lived longer whilebreathingit. After breathing the gas, Priestley said that it felt like normal air, but his lungs felt lighter and easy afterwards.[7]His findings were published in 1775.[4][15]It is because his findings were published first that he is often said to have discovered oxygen.
FrenchchemistAntoine Lavoisierlater said he had discovered the substance as well. Priestley visited him in 1774 and told him about his experiment. Scheele also sent a letter to Lavoisier in that year that spoke of his discovery.[14]
Lavoisier's research[change|change source]

Lavoisier did the first main experiments onoxidation.He was the first person to explain how combustion works.[13]He used these and other experiments to prove the phlogiston theory wrong. He also tried to prove that the substance discovered by Priestley and Scheele was achemical element.
In one experiment, Lavoisier found that there was no increase inweightwhentinand air were heated in a closedcontainer.He also found that air rushed in when the container was opened. After this, he found that the weight of the tin had increased by the same amount as the weight of the air that rushed in. He published his findings in 1777.[13]He wrote that air was made up of two gases. One he called "vital air" (oxygen), which is needed for combustion and respiration. The other (nitrogen) he called "azote", which means "lifeless" inGreek.(This is still the name of nitrogen in some languages, includingFrench.)[13]
Lavoisier renamed "vital air" to "oxygène", from Greek words meaning "sour making" or "producer ofacid".He called it this because he thought oxygen was in all acids, which is wrong.[16]Later chemists realised that Lavoiser's name for the gas was wrong, but the name was too common by then to change.[17]
"Oxygen" became the name in theEnglish language,even though English scientists were against it.
Later history[change|change source]
John Dalton's theory ofatomssaid that all elements had one atom and atoms in compounds were usually alone. For example, he wrongly thought that water (H2O) had the formula of just HO.[18]In 1805,Joseph Louis Gay-LussacandAlexander von Humboldtshowed that water is made up of two hydrogen atoms and one oxygen atom. By 1811,Amedeo Avogadrocorrectly worked out what water was made of based onAvogadro's law.[19]
By the late 19th century, scientists found that air could be turned into a liquid and the compounds in it could be isolated bycompressingand cooling it.Swisschemist and physicistRaoul Pictetdiscoveredliquidoxygen byevaporatingsulfur dioxideto turncarbon dioxideinto a liquid. This was then also evaporated to cool oxygen gas in order to turn it into a liquid. He sent atelegramto theFrench Academy of Scienceson 22 December 1877 telling them of his discovery.[20]
Characteristics[change|change source]
Properties and molecular structure[change|change source]
Atstandard temperature and pressure,oxygen has nocolour,odourortaste.It is a gas with thechemical formulaO
2called dioxygen.[21]
Asdioxygen(or justoxygen gas), two oxygenatomsarechemically boundto each other. This bond can be called many things, but simply called acovalentdouble bond.Oxygen gas is veryreactiveand can react with many other elements.Oxidesare made whenmetalelements react with oxygen, such asiron oxide,which is known asrust.There are a lot of oxide compounds on Earth.
Allotropes[change|change source]
The commonallotrope(type) of oxygen on Earth is called dioxygen (O2). This is the second biggest part of the Earth's atmosphere, after dinitrogen (N2). O2has a bond length of 121pmand a bondenergyof 498kJ/mol[22]Because of its energy, O2is used by complex life likeanimals.
Ozone(O3) is very reactive and damages thelungswhen breathed in.[23]Ozone is made in the upperatmospherewhen O2combines with pure oxygen made when O2is split byultraviolet radiation.[16]Ozone absorbs a lot of radiation in the UV part of theelectromagnetic spectrumand so theozone layerin the upper atmosphere protects Earth from radiation.
Above the ozone layer, (inlow Earth orbits), atomic oxygen becomes the most common form.[24]
Tetraoxygen(O4) was discovered in 2001.[25][26]It only exists in extreme conditions when a lot ofpressureis put onto O2.
Physical properties[change|change source]
Oxygendissolvesmore easily from air into water thannitrogendoes. When there is the same amount of air and water, there is one molecule of O2for every 2 molecules of N2(aratioof 1:2). This is different to air, where there is a 1:4 ratio of oxygen to nitrogen. It is also easier for O2to dissolve infreshwaterthan inseawater.[7][27]Oxygencondensesat 90.20K(-182.95°C,-297.31 °F) andfreezesat 54.36 K (-218.79 °C, -361.82 °F).[28]Bothliquidand solid O2are see-through with a light-blue colour.
Oxygen is very reactive and must be kept away from anything that can burn.[29]
Isotopes[change|change source]
There are three stableisotopesof oxygen in nature. They are16O,17O, and18O. About 99.7% of oxygen is the16O isotope.[30]
Occurrence[change|change source]
Oxygen is the third most common element in the universe, afterhydrogenand helium.[31]About 0.9% of theSun's mass is oxygen.[13]
| Z | Element | Mass fraction in parts per million | ||
|---|---|---|---|---|
| 1 | Hydrogen | 739,000 | 71 × mass of oxygen (red bar) | |
| 2 | Helium | 240,000 | 23 × mass of oxygen (red bar) | |
| 8 | Oxygen | 10,400 | ||
| 6 | Carbon | 4,600 | ||
| 10 | Neon | 1,340 | ||
Apart fromiron,oxygen is the most common element on Earth (by mass). It makes up nearly half (46%
to 49.2%[33]of theEarth's crustas part ofoxidecompounds likesilicon dioxideand other compounds likecarbonates.It is also the main part of the Earth'soceans,making up 88.8% by mass. Oxygen gas is the second most common part of the atmosphere, making up 20.95%[34]of its volume and 23.1% of its volume. Earth is strange compared to otherplanets,as a large amount of its atmosphere is oxygen gas.Marshas only 0.1%O
2by volume, and the other planets have less than that.
The much higher amount of oxygen gas around Earth is caused by the oxygen cycle.Photosynthesistakes hydrogen fromwaterusingenergy from sunlight.This gives off oxygen gas. Some of the hydrogen combines withcarbon dioxideto makecarbohydrates.Respirationthen takes oxygen gas out of the atmosphere or water and turns it into carbon dioxide and water. [35]
Uses[change|change source]

Medical[change|change source]
O2is a very important part ofrespiration.Because of this, it is used in medicine. It is used to increase the amount of oxygen in a personsbloodso more respiration can take place. This can make them become healthy quicker if they are ill.Oxygen therapyis used to treatemphysema,pneumonia,someheartproblems, and anydiseasethat makes it harder for a person to take in oxygen.[36]
Life support[change|change source]
Low-pressure O2is used inspace suits,surrounding the body with the gas. Pure oxygen is used but at a much lower pressure. If the pressure were higher, it would be poisonous.[37][38]
Industrial[change|change source]
Smeltingofiron oreintosteeluses about 55% of oxygen made by humans.[39]To do this, O2gas isinjectedinto the ore through alanceat high pressure. This removes anysulfurorcarbonfrom the ore that would not be wanted. They are given off assulfur oxideandcarbon dioxide.The temperature can go as high as 1,700 °Cbecause it is anexothermic reaction.[39]
Around 25% of oxygen made by humans is used by chemists.[39]Ethyleneis reacted with O2to makeethylene oxide.This is then changed toethylene glycol,which is used to make many products such asantifreezeandpolyester(these can then be turned intoplasticsandfabrics).[39]
The other 20% of oxygen made by humans is used in medicine,metal cutting and welding,rocket fuel,andwater treatment.[39]
Compounds[change|change source]
Theoxidation stateof oxygen is −2 in nearly everycompoundit is in. In a few compounds, the oxidation state is −1, such asperoxides.Compounds of oxygen with other oxygen states are very uncommon.[40]
Oxides and other inorganic compounds[change|change source]
Water(H
2O) is anoxideofhydrogen.It is the most common oxide on Earth. All knownlifeneeds water to live. Water is made of two hydrogen atomscovalent bondedto an oxygen atom (oxygen has a higherelectronegativitythan hydrogen).[41](this is the basic principle of covalent bonding)
There are also electrostatic forces (Van de'r Waals forces) between the hydrogen atoms and adjacent molecules' oxygen atoms. These pseudo-bonds bring the atoms around 15% closer to each other than most other simple liquids. This is because Water is apolar molecule(Net asymmetrical distribution of electrons) due to its bent shape, giving it an overall net field direction, mainly due to oxygens 2 non bonding pairs of electrons, pushing the bonding H's further together than the linear arrangement with lower enthalpy (see CO2). This property is exploited by microwaves to oscillate polar molecules, especially water. And its responsible for the extra energy needed to disassociate H20.[42]
Because of oxygen's high electronegativity, it makeschemical bondswith almost all other chemical elements. These bonds giveoxides(for exampleironreacts with oxygen to giveiron oxide). Mostmetal's surfaces are turned into oxides when inair.Iron's surface will turn torust(iron oxide) when in air for a long time. There are small amounts ofcarbon dioxide(CO
2) in the air, and it is turned intocarbohydratesduringphotosynthesis.Living things give it off duringrespiration.[43]
Organic compounds[change|change source]
Manyorganic compoundshave oxygen in them. Some of the classes of organic compounds that have oxygen arealcohols,ethers,ketones,aldehydes,carboxylic acids,esters,andamides.Many organicsolventsalso have oxygen, such asacetone,methanol,andisopropanol.Oxygen is also found in nearly allbiomoleculesthat are made by living things.
Oxygen also reacts quickly with many organic compounds at, or below,room temperaturewhenautoxidationhappens.[44]
Industrial production[change|change source]

One hundred million tonnes of O2are gotten from air for industrial uses every year. Industries use two main methods to make oxygen. The most common method isfractional distillationofliquefied air.N2evaporateswhile O2is left as aliquid.[7]O2is the second most important industrialgas.Because it is more economical, oxygen is usually stored and transported as aliquid.A small steel tank of 16literswater capacity with a workingpressureof 139bar(2015 psi) holds about 2150 liters of gas and weighs 28kilograms(62 lb) empty. 2150 liters of oxygen weighs about 3 kilograms (6.6 lb).
The other main method of making oxygen is by passing a stream of clean, dry air through a pair ofzeolitemolecular sieves.The zeolite molecular sieves soaks up the nitrogen. It gives a stream of gas that is 90% to 93% oxygen.[7]
Oxygen gas can also be made throughelectrolysis of waterinto molecular oxygen andhydrogen.[7]
Safety[change|change source]
Oxygen'sNFPA 704says that compressed oxygen gas is not dangerous to health and is not flammable.[45]
Toxicity[change|change source]
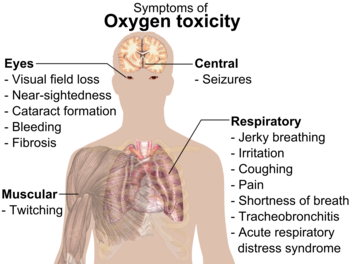
At highpressures,oxygen gas (O2) can be dangerous toanimals,includinghumans.It can causeconvulsionsand otherhealthproblems.[a][46]Oxygentoxicityusually begins to occur at pressures more than 50 kilopascals(kPa), equal to about 50% oxygen in the air at standard pressure (air on Earth has around 20% oxygen).[7]
Premature babiesused to be placed in boxes with air with a high amount of O2.This was stopped when some babies wentblindfrom the oxygen.[7]
Breathing pure O2in space suits causes no damage because there is a lower pressure used.[47]
Combustion and other hazards[change|change source]
Concentrated amounts of pure O2can cause a quickfire.When concentrated oxygen andfuelsare brought close together, a slightignitioncan cause a huge fire.[48]TheApollo 1crew were all killed by a fire because the air of the capsule had a very high amount of oxygen.[b][50]
If liquid oxygen is spilled ontoorganic compounds,likewood,it canexplode.[48]
Related pages[change|change source]
References[change|change source]
- ↑"Standard Atomic Weights: Oxygen".CIAAW.2009.
- ↑Weast, Robert (1984).CRC, Handbook of Chemistry and Physics.Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110.ISBN0-8493-0464-4.
- ↑Jastrow, Joseph (1936).Story of Human Error.Ayer Publishing. p. 171.ISBN978-0-8369-0568-7.
- ↑4.04.14.24.34.4Cook, Gerhard A. & Lauer, Carol M. 1968. "Oxygen". In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 499–512. LCCN 68-29938. p499.
- ↑Chisholm, Hugh, ed. (1911)..Encyclopædia Britannica.Vol. 17 (11th ed.). Cambridge University Press. pp. 938–939.
- ↑"John Mayow".World of Chemistry.Thomson Gale. 2005.ISBN978-0-669-32727-4.RetrievedDecember 16,2007.
- ↑7.07.17.27.37.47.57.67.7Emsley 2001,p.299
- ↑Best, Nicholas W. (2015). "Lavoisier's 'Reflections on Phlogiston' I: Against Phlogiston Theory".Foundations of Chemistry.17(2): 137–151.doi:10.1007/s10698-015-9220-5.S2CID170422925.
- ↑Morris, Richard (2003).The last sorcerers: The path from alchemy to the periodic table.Washington, D.C.: Joseph Henry Press.ISBN978-0-309-08905-0.
- ↑Marples, Frater James A."Michael Sendivogius, Rosicrucian, and father of studies of oxygen"(PDF).Societas Rosicruciana in Civitatibus Foederatis, Nebraska College. pp. 3–4.RetrievedMay 25,2018.
- ↑Bugaj, Roman (1971)."Michał Sędziwój - Traktat o Kamieniu Filozoficznym".Biblioteka Problemów(in Polish).164:83–84.ISSN0137-5032.
- ↑"Oxygen".RSC.org.RetrievedDecember 12,2016.
- ↑13.013.113.213.313.413.5Cook & Lauer 1968,p. 500
- ↑14.014.1Emsley 2001,p. 300
- ↑Priestley, Joseph (1775). "An account of further discoveries in air".Philosophical Transactions.65:384–94.doi:10.1098/rstl.1775.0039.S2CID186214794.
- ↑16.016.1Parks, G. D.; Mellor, J. W. (1939).Mellor's Modern Inorganic Chemistry(6th ed.). London: Longmans, Green and Co.
- ↑Greenwood & Earnshaw, pg. 793
- ↑DeTurck, Dennis; Gladney, Larry; Pietrovito, Anthony (1997)."Do We Take Atoms for Granted?".The Interactive Textbook of PFP96.University of Pennsylvania. Archived fromthe originalon January 17, 2008.RetrievedJanuary 28,2008.
- ↑Roscoe, Henry Enfield; Schorlemmer, Carl (1883).A Treatise on Chemistry.D. Appleton and Co. p. 38.
- ↑Daintith, John (1994).Biographical Encyclopedia of Scientists.CRC Press. p. 707.ISBN978-0-7503-0287-6.
- ↑"Oxygen Facts".Science Kids. February 6, 2015.RetrievedNovember 14,2015.
- ↑Chieh, Chung."Bond Lengths and Energies".University of Waterloo. Archived fromthe originalon December 14, 2007.RetrievedDecember 16,2007.
- ↑Stwertka, Albert (1998).Guide to the Elements(Revised ed.). Oxford University Press. pp.48–49.ISBN978-0-19-508083-4.
- ↑"Atomic oxygen erosion".Archived fromthe originalon June 13, 2007.RetrievedJuly 2,2020.
atomic oxygen, the major component of the low Earth orbit environment
- ↑Cacace, Fulvio; de Petris, Giulia; Troiani, Anna (2001). "Experimental Detection of Tetraoxygen".Angewandte Chemie International Edition.40(21): 4062–65.doi:10.1002/1521-3773(20011105)40:21<4062::AID-ANIE4062>3.0.CO;2-X.PMID12404493.
- ↑Ball, Phillip (September 16, 2001)."New form of oxygen found".Nature News.RetrievedJanuary 9,2008.
- ↑"Air solubility in water".The Engineering Toolbox.RetrievedDecember 21,2007.
- ↑Lide, David R. (2003). "Section 4, Properties of the elements and inorganic Compounds; melting, boiling, and critical temperatures of the elements".CRC Handbook of Chemistry and Physics(84th ed.). Boca Raton, Florida: CRC Press.ISBN978-0-8493-0595-5.
- ↑"Liquid Oxygen Material Safety Data Sheet"(PDF).Matheson Tri Gas. Archived fromthe original(PDF)on February 27, 2008.RetrievedDecember 15,2007.
- ↑"Oxygen Nuclides / Isotopes".EnvironmentalChemistry.RetrievedDecember 17,2007.
- ↑Emsley 2001,p.297
- ↑Croswell, Ken (February 1996).Alchemy of the Heavens.Anchor.ISBN978-0-385-47214-2.
- ↑ "Oxygen".Los Alamos National Laboratory. Archived fromthe originalon October 26, 2007.RetrievedDecember 16,2007.
- ↑Mackenzie, F.T. and J.A. (1995)."Gaseous Composition of Dry Air".Our changing planet.Prentice-Hall. pp. 288–307. Archived fromthe originalon 2020-04-13.Retrieved2020-07-02.
20.947
- ↑Canfield, Donald 2014.Oxygen: a four billion year history.Princeton: Princeton University Press.ISBN 978-0-691-14502-0
- ↑Cook & Lauer 1968,p.510
- ↑Morgenthaler GW; Fester DA; Cooley CG (1994)."As assessment of habitat pressure, oxygen fraction, and EVA suit design for space operations".Acta Astronautica.32(1): 39–49.Bibcode:1994AcAau..32...39M.doi:10.1016/0094-5765(94)90146-5.PMID11541018.
- ↑Webb JT; Olson RM; Krutz RW; Dixon G; Barnicott PT (1989). "Human tolerance to 100% oxygen at 9.5 psia during five daily simulated 8-hour EVA exposures".Aviat Space Environ Med.60(5): 415–21.doi:10.4271/881071.PMID2730484.
- ↑39.039.139.239.339.4Emsley 2001, p.301
- ↑IUPAC:Red Book.p. 73 and 320.
- ↑Chaplin, Martin (January 4, 2008)."Water Hydrogen Bonding".RetrievedJanuary 6,2008.
- ↑Maksyutenko, P.; Rizzo, T. R.; Boyarkin, O. V. (2006). "A direct measurement of the dissociation energy of water".J. Chem. Phys.125(18): 181101.Bibcode:2006JChPh.125r1101M.doi:10.1063/1.2387163.PMID17115729.
- ↑Smart, Lesley E.; Moore, Elaine A. (2005).Solid State Chemistry: an introduction(3rd ed.). CRC Press. p.214.ISBN978-0-7487-7516-3.
- ↑Cook & Lauer 1968, p.506
- ↑"NFPA 704 ratings and id numbers for common hazardous materials"(PDF).Riverside County Department of Environmental Health. Archived fromthe original(PDF)on July 11, 2019.RetrievedAugust 22,2017.
- ↑Cook & Lauer 1968,p.511
- ↑Wade, Mark (2007)."Space Suits".Encyclopedia Astronautica. Archived fromthe originalon December 13, 2007.RetrievedDecember 16,2007.
- ↑48.048.1Werley, Barry L, ed. (1991).ASTM Technical Professional training.Fire hazards in oxygen systems.Philadelphia: ASTM International Subcommittee G-4.05.
- ↑Report of Apollo 204 Review Board NASA Historical Reference Collection, NASA History Office, NASA HQ, Washington DC
- ↑Chiles, James R. (2001).Inviting Disaster: lessons from the edge of technology: an inside look at catastrophes and why they happen.New York: HarperCollins Publishers Inc.ISBN978-0-06-662082-4.
- ↑SinceO
2's partial pressure is the fraction ofO
2times the total pressure, elevated partial pressures can occur either from highO
2fraction in breathing gas or from high breathing gas pressure, or a combination of both. - ↑No single ignition source of the fire was conclusively identified, although some evidence points to an arc from an electrical spark.[49]
General references[change|change source]
- Emsley, John (2001)."Oxygen".Nature's building blocks: An A-Z guide to the elements.Oxford, England: Oxford University Press. pp.297–304.ISBN978-0-19-850340-8.
- Canfield, Donald 2014.Oxygen: a four billion year history.Princeton: Princeton University Press.ISBN 978-0-691-14502-0
- Lane, Nick 2002.Oxygen: the molecule that made the world.Oxford University Press.ISBN 0-19-860783-0
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||||||||||
| |||||||||||||||||||||||||||||||||||||||||


