Medical Device recall notification (U.S. only) / field safety notice (International Markets)
Philips Respironics Sleep and Respiratory Care devices
In June 2021, after discovering a potential health risk related to a part in certain CPAP, BiPAP and Mechanical Ventilator devices, Philips issued a voluntary Field Safety Notice (International markets) / voluntary recall notification (U.S. only). Patient safety is our top priority, and we are committed to supporting our patients, durable medical equipment providers (DMEs), distributors, home health partners, and clinicians through the complete remediation process. Throughout the remediation of this field safety notice, we will provide guidance and share next steps so you can have the most current and accurate information. We thank you for your patience as we work to restore your trust.
If you use one of the affected devices, the Medical Device recall notification (U.S. only) / field safety notice (International Markets) advises patients and customers to take the following actions:

Continue life-sustaining ventilation prescribed therapy
Consult with your physician as soon as possible to determine appropriate next steps.
Stop use of BiLevel PAP & CPAP sleep apnea devices
Consult with your physician to determine the benefits of continuing therapy and potential risks.
There is nothing we take more seriously than providing patients with high quality products that are safe and reliable. If an issue arises, we are proactive in communicating and addressing it as we work tirelessly towards a resolution.
Latest information
The remediation of this field safety notice is underway and has started for the following devices: • DreamStation CPAPs • DreamStation BiPAPs • DreamStation ST/AVAPS Philips aims to address all affected devices within the scope of this field safety notice but due to the volume of devices that have been affected, we regret it may take some time to repair or replace patients' devices. Philips will provide further updates on the remediation of this field safety notice, including updates on other affected models.

Philips CEO Frans van Houten and Chief Business Leader Connected Care Roy Jakobs talk about the various aspects of the field safety notice

Technical Project Manager Jan Bennik speaks about the test and research program
What you need to do
Philips is committed to rectfying this issue through a robust and comprehensive repair and replacement program. We have established a claims processing and support center to assist you.

Durable Medical Equipment Providers, Distributors, or Medical Institutions
Click the link below to begin our registration process. You should have received a letter from Philips about this issue that contains log-in credentials for the registration website. If you do not have this letter, please call the number below. After registration, we will notify you with additonal information as it becomes available.
9am-6pm (Mon-Fri)
Downloadable resources

Patients, Users, or Caregivers
9am-6pm (Mon-Fri)
What devices are affected by the recall notification (U.S. only) / field safety notice (International Markets)?
The recall notification (U.S. only) / field safety notice (International Markets) provides customers with information on how to identify affected products. Additionally, the device Instructions for Use provide product identification information to assist with this activity. Products affected by this Medical Device recall notification (U.S. only) / field safety notice (International Markets):
CPAP and BiLevel PAP Devices
All Affected Devices Manufactured Before 26 April 2021, All Device Serial Numbers

Stop use of BiLevel PAP & CPAP sleep apnea devices
Consult with your physician to determine the benefits of continuing therapy and potential risks.
Continuous Ventilator, Minimum Ventilatory Support, Facility Use
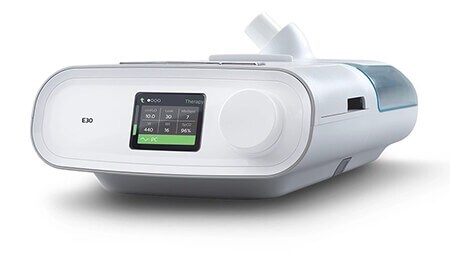
E30
(Emergency Use Authorization)
Continuous Ventilator, Non-life Supporting
Remediation in progress

DreamStation
ASV
Remediation in progress

DreamStation
ST, AVAPS

SystemOne
ASV4

C Series
ASV, S/T, AVAPS

OmniLab Advanced Plus
In-Lab Titration Device
Non-continuous Ventilator

SystemOne
(Q series)
Remediation in progress

DreamStation
CPAP, Auto CPAP, BiPAP

DreamStation GO
CPAP, APAP

Dorma 400, 500
CPAP

REMStar SE Auto
CPAP
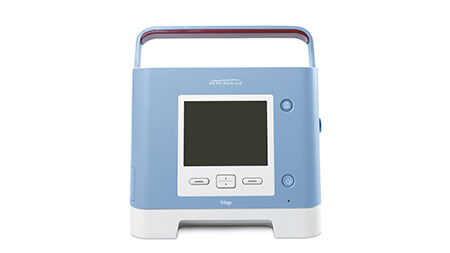
Trilogy 100
Ventilator

Trilogy 200
Ventilator

Garbin Plus, Aeris, LifeVent
Ventilator
Continuous Ventilator, Minimum Ventilatory Support, Facility Use

A-Series BiPAP Hybrid A30
(not marketed in US)

A-Series BiPAP V30 Auto
Ventilator
Continuous Ventilator, Non-life Supporting

A-Series BiPAP A40
(not marketed in US)

A-Series BiPAP A30
(not marketed in US)
What products are not affected and why?
Products that are not affected may have different sound abatement foam materials, as new materials and technologies are available over time. Also, sound abatement foam in unaffected devices may be placed in a different location due to device design. Products not affected by this recall notification (U.S. only) / field safety notice (International Markets) include:
Questions and answers
- Are affected devices safe for use? Should affected devices be removed from service?
The recall notification (U.S. only) / field safety notice (International Markets) advises patients and customers to take the following actions:
- For patients using BiLevel PAP and CPAP devices:Discontinue use of affected units and consult with physicians to determine the benefits of continuing therapy and potential risks.
- For patients using life-sustaining mechanical ventilator devices:DO NOT discontinue or alter prescribed therapy,without consulting physicians to determine appropriate next steps.
Philips is recommending that customers and patients halt use of ozone-related cleaning products, and adhere to their device Instructions for Use for approved cleaning methods.
Additionally, Philips is reminding customers and patients to review the age of their BiLevel PAP and CPAP devices, as they are typically recommended to be replaced after five years of use.
- What is the safety hazard associated with this issue? Has Philips received any reports of patient harm due to this issue?
The recall notification (U.S. only) / field safety notice (International Markets) informs customers and users of potential impacts on patient health and clinical use related to this issue. Possible health risks include exposure to degraded sound abatement foam, for example caused by unapproved cleaning methods such as ozone, and exposure to chemical emissions from the foam material. High heat and high humidity environments may also contribute to foam degradation in certain regions.
Philips continues to monitor reports of potential safety issues through our post-market surveillance activities as required by medical device regulations and laws in the markets in which we operate.
In the event of exposure to degraded foam:
- The potential risks of degraded foam exposure include:
- Irritation (skin, eye, and respiratory tract), inflammatory response, headache, asthma, adverse effects to other organs (e.g. kidneys and liver) and toxic carcinogenic affects.
- To date, Philips Respironics has received several complaints regarding the presence of black debris/particles within the airpath circuit (extending from the device outlet, humidifier, tubing, and mask). Philips also has received reports of headache, upper airway irritation, cough, chest pressure and sinus infection.
In the event of exposure to chemical emissions:
- The potential risks of exposure due to chemical emissions from affected foam include: headache/dizziness, irritation (eyes, nose, respiratory tract, skin), hypersensitivity, nausea/vomiting, toxic and carcinogenic effects.
- To date, Philips has not received reports of patient impact or serious harm as a result of this issue.
- The potential risks of degraded foam exposure include:
- When will the correction for this issue begin? How long will it take to address all affected devices?
Philips is notifying regulatory agencies in the regions and countries where affected products are available.
We are providing agencies with required information related to the initial launch and ongoing implementation of the projected correction.
The company will replace the current sound abatement foam with a new material that is not affected by this issue, and has already begun this process.
At this time, the company is working to address all affected devices within the scope of this correction as expeditiously as possible.
- Are affected devices continuing to be manufactured and/or shipped?
At this time, affected devices are on manufacturing and ship hold as the company prepares to implement the repair / replacement program for affected devices, to install new sound abatement foam material not affected by the reported issues.
- Is this a recall? Have regulatory authorities classified the severity of the recall?
The issuance of the notification is a recall in the U.S., and field safety notice in International Markets, according to regulatory agency criteria.
This recall notification / field safety notice has not yet been classified by regulatory agencies.
- How will Philips address this issue? Are affected devices being replaced and/or repaired? Are customers entitled to warranty replacement, repair, service or other mitigations?
We are treating this matter with the highest possible seriousness, and are working to address this issue as efficiently and thoroughly as possible.
As a result of extensive ongoing analysis, on June 14, 2021, the company issued a recall notification for specific affected Continuous Positive Airway Pressure (CPAP), Bi-Level Positive Airway Pressure (BiLevel PAP) devices, and Mechanical Ventilators.
The recall notification (U.S. only) / field safety notice (International Markets) informs customers and users of potential impacts on patient health and clinical use related to this issue. Possible health risks include exposure to degraded sound abatement foam, for example caused by unapproved cleaning methods such as ozone, and exposure to chemical emissions from the foam material.
Philips is notifying customers and users of affected devices that the company will replace the current sound abatement foam with a new material that is not affected by this issue. Affected devices currently will be either replaced with a new or refurbished unit that incorporates the new material, or repaired to replace the sound abatement foam in customer units. The new material will also replace the current sound abatement foam in future products.
Philips is recommending that customers and patients halt use of ozone-related cleaning products, and adhere to their device Instructions for Use for approved cleaning methods.
Additionally, Philips is reminding customers and patients to review the age of their BiLevel PAP and CPAP devices, as they are typically recommended to be replaced after five years of use.
The company has dedicated significant resources to address this issue, and has developed a comprehensive plan for this correction, and has already begun this process. This effort includes wide-scale, global ramping up of manufacturing, repair, services, supply chain and other functions to support the correction.
Philips deeply regrets the inconveniences caused by this issue, and we are dedicating significant time and resources to give affected patients and customers the service they expect and deserve as we resolve this matter as our top priority.
For more information on the recall notification, as well as instructions for customers, users and physicians, affected parties may contact their local Philips representative or visitwww.philips.com/SRC-update.Call 1800-28-63-020 if you cannot visit the website or do not have internet access.
- Are there any steps that customers, patients, users and/or clinicians should take regarding this issue?
Customers, patients, users and clinicians are instructed to follow the guidance contained in the recall notification (U.S. only) / field safety notice (International Markets).
The recall notification (U.S. only) / field safety notice (International Markets) advises patients and customers to take the following actions:
- For patients using BiLevel PAP and CPAP devices:Discontinue use of affected units and consult with physicians to determine the benefits of continuing therapy and potential risks.
- For patients using life-sustaining mechanical ventilator devices:DO NOT discontinue or alter prescribed therapy,without consulting physicians to determine appropriate next steps.
- Register affected devices on the recall notification (U.S. only) / field safety notice (International Markets),www.philips.com/SRC-update.
- The website provides current information on the status of the recall notification (U.S. only) / field safety notice (International Markets) and how to receive permanent corrective action to address the two issues.
- The website also provides instructions on how to locate an affected device Serial Number and will guide users through the registration process.
- Call 1800-28-63-020 if you cannot visit the website or do not have internet access.
The company has developed a comprehensive plan for this correction, and has already begun this process.
Philips is recommending that customers and patients halt use of ozone-related cleaning products, and adhere to their device Instructions for Use for approved cleaning methods.
Additionally, Philips is reminding customers and patients to review the age of their BiLevel PAP and CPAP devices, as they are typically recommended to be replaced after five years of use.
Philips deeply regrets the inconveniences caused by this issue, and we are dedicating significant time and resources to give affected patients and customers the service they expect and deserve as we resolve this matter as our top priority.
For more information on the recall notification (U.S. only) / field safety notice (International Markets), as well as instructions for customers, users and physicians, affected parties may contact their local Philips representative or visitwww.philips.com/SRC-update.Call 1800-28-63-020 if you cannot visit the website or do not have internet access.
- What is the cause of this issue? Was it a design, manufacture, supplier or other problem?
Based on Philips analysis, the root cause of this issue is related to the sound abatement foam currently used in specific identified products of the Sleep & Respiratory Care portfolio.
- How did this happen, and what is Philips doing to ensure it will not happen again?
Philips has a robust Quality Management System and has followed our review and analysis processes to help identify and address this issue.
The products were designed according to, and in compliance with, appropriate standards upon release. As new standards are developed, they require assessment of product characteristics according to quality and regulatory processes. Philips Quality Management System has been updated to reflect these new requirements.
However, while standards have been updated, products developed on the prior standard are still in compliance with medical device regulations. The foam degradation and chemical emission issues were discovered as part of our Quality Management System processes, and are being corrected in accordance with appropriate regulatory requirements.
Philips has been in full compliance with relevant standards upon product commercialization.
- What is meant by "high heat and humidity" being one of the causes of this issue?
Philips has determined that the foam may degrade under certain circumstances, influenced by factors including use of unapproved cleaning methods, such as ozone), and certain environmental conditions involving high humidity and temperature.
The environmental conditions that may be one of the causes of this issue refer to the climate and regional temperatures of the countries where the devices are used and stored.
This factor does not refer to heat and humidity generated by the device for patient use.
- Do affected units exhibit features that customers / users should watch out for? Particles or other visible issues?
Users should consult with their physicians as directed in the recall notification (U.S. only) / field safety notice (International Markets).
- Can Philips replace products under warranty or repair devices under warranty?
Affected devices may be repaired under warranty.
Philips will provide further information regarding warranty replacement procedures during this issue when it is available.
- In those regions where Philips provides both patient care and devices, will new patients be set up with devices? Will existing patient devices that fail be replaced?
At this time, Philips is unable to set up new patients on affected devices. Philips may work with new patients to provide potential alternate devices.
Philips may repair / replace ventilator units that patients are reliant on in emergency situations such as device failure during required treatment, to ensure continuity of care.
Philips CPAPs cannot be replaced during ship hold.
- Is Philips certain that this issue is limited to the listed devices? Is there any possibility others are affected?
Philips has completed our analysis in accordance with our Quality Management System and identified all affected products, which are included in our notifications to regulatory agencies and customers.
No further products are affected by this issue.
